Automated Abdominal CT Imaging Biomarkers for Opportunistic Prediction of Future Major Osteoporotic Fractures in Asymptomatic Adults
- PMID: 32780005
- PMCID: PMC7526945
- DOI: 10.1148/radiol.2020200466
Automated Abdominal CT Imaging Biomarkers for Opportunistic Prediction of Future Major Osteoporotic Fractures in Asymptomatic Adults
Abstract
Background Body composition data from abdominal CT scans have the potential to opportunistically identify those at risk for future fracture. Purpose To apply automated bone, muscle, and fat tools to noncontrast CT to assess performance for predicting major osteoporotic fractures and to compare with the Fracture Risk Assessment Tool (FRAX) reference standard. Materials and Methods Fully automated bone attenuation (L1-level attenuation), muscle attenuation (L3-level attenuation), and fat (L1-level visceral-to-subcutaneous [V/S] ratio) measures were derived from noncontrast low-dose abdominal CT scans in a generally healthy asymptomatic adult outpatient cohort from 2004 to 2016. The FRAX score was calculated from data derived from an algorithmic electronic health record search. The cohort was assessed for subsequent future fragility fractures. Subset analysis was performed for patients evaluated with dual x-ray absorptiometry (n = 2106). Hazard ratios (HRs) and receiver operating characteristic curve analyses were performed. Results A total of 9223 adults were evaluated (mean age, 57 years ± 8 [standard deviation]; 5152 women) at CT and were followed over a median time of 8.8 years (interquartile range, 5.1-11.6 years), with documented subsequent major osteoporotic fractures in 7.4% (n = 686), including hip fractures in 2.4% (n = 219). Comparing the highest-risk quartile with the other three quartiles, HRs for bone attenuation, muscle attenuation, V/S fat ratio, and FRAX were 2.1, 1.9, 0.98, and 2.5 for any fragility fracture and 2.0, 2.5, 1.1, and 2.5 for femoral fractures, respectively (P < .001 for all except V/S ratio, which was P ≥ .51). Area under the receiver operating characteristic curve (AUC) values for fragility fracture were 0.71, 0.65, 0.51, and 0.72 at 2 years and 0.63, 0.62, 0.52, and 0.65 at 10 years, respectively. For hip fractures, 2-year AUC for muscle attenuation alone was 0.75 compared with 0.73 for FRAX (P = .43). Multivariable 2-year AUC combining bone and muscle attenuation was 0.73 for any fragility fracture and 0.76 for hip fractures, respectively (P ≥ .73 compared with FRAX). For the subset with dual x-ray absorptiometry T-scores, 2-year AUC was 0.74 for bone attenuation and 0.65 for FRAX (P = .11). Conclusion Automated bone and muscle imaging biomarkers derived from CT scans provided comparable performance to Fracture Risk Assessment Tool score for presymptomatic prediction of future osteoporotic fractures. Muscle attenuation alone provided effective hip fracture prediction. © RSNA, 2020 See also the editorial by Smith in this issue.
Figures

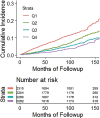
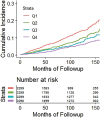
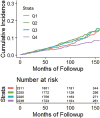
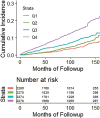
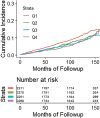
![Receiver operating characteristic (ROC) curves for predicting future fragility fractures. (a) ROC curves for predicting any fragility fracture over a 2-year time horizon shows comparable performance between the univariable L1-bone attenuation (area under the ROC curve [AUC] = 0.71) and the multivariable Fracture Risk Assessment Tool (FRAX) (AUC = 0.72), whereas visceral-to-subcutaneous (V/S) fat ratio was a poor predictor (AUC = 0.51). When bone attenuation and muscle attenuation are combined, the performance improves slightly (AUC = 0.73). (b) ROC curves for predicting hip fractures over a 2-year time horizon show that the univariable muscle attenuation alone (AUC = 0.75) compares favorably with the multivariable FRAX (AUC = 0.73). When bone attenuation and muscle attenuation are combined (not shown), the performance is further improved, albeit only slightly (AUC = 0.76).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/2f61/7526945/0701d637bcdf/radiol.2020200466.fig3a.gif)
![Receiver operating characteristic (ROC) curves for predicting future fragility fractures. (a) ROC curves for predicting any fragility fracture over a 2-year time horizon shows comparable performance between the univariable L1-bone attenuation (area under the ROC curve [AUC] = 0.71) and the multivariable Fracture Risk Assessment Tool (FRAX) (AUC = 0.72), whereas visceral-to-subcutaneous (V/S) fat ratio was a poor predictor (AUC = 0.51). When bone attenuation and muscle attenuation are combined, the performance improves slightly (AUC = 0.73). (b) ROC curves for predicting hip fractures over a 2-year time horizon show that the univariable muscle attenuation alone (AUC = 0.75) compares favorably with the multivariable FRAX (AUC = 0.73). When bone attenuation and muscle attenuation are combined (not shown), the performance is further improved, albeit only slightly (AUC = 0.76).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/2f61/7526945/bbe3f942df8f/radiol.2020200466.fig3b.gif)

Comment in
-
Automated Screening for Future Osteoporotic Fractures on Abdominal CT: Opportunistic or an Outstanding Opportunity?Radiology. 2020 Oct;297(1):73-74. doi: 10.1148/radiol.2020202900. Epub 2020 Aug 11. Radiology. 2020. PMID: 32956028 No abstract available.
References
-
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 2007;22(3):465–475. - PubMed
-
- Roux C, Briot K. The crisis of inadequate treatment in osteoporosis. Lancet Rheumatol 2020;2(2):e110–e119. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

