Advances in research on ACE2 as a receptor for 2019-nCoV
- PMID: 32780149
- PMCID: PMC7417784
- DOI: 10.1007/s00018-020-03611-x
Advances in research on ACE2 as a receptor for 2019-nCoV
Abstract
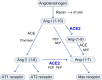
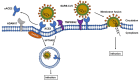
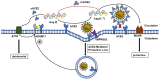
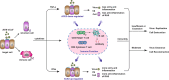
Currently, a novel coronavirus (SARS-CoV-2, also called 2019-nCoV) has triggered pandemic Coronavirus Disease 2019 (COVID-19), an acute infectious respiratory disease that first became epidemic in Wuhan (China) and is now spreading worldwide. Although 2019-nCoV and SARS-CoV are very similar viruses genomically and structurally, the huge number of severe cases and deaths now being caused by 2019-nCoV infections has understandably prompted intense research on the receptor used by it to enter human cells. Angiotensin converting enzyme 2 (ACE2), a functional receptor for SARS-CoV, now appears likely to mediate 2019-nCoV entry into human cells. In this review, we describe the roles performed by ACE2 as an enzymatic catalyst and as a receptor for this novel coronavirus. We also summarize the latest research pertaining to the changes noted in ACE2 expression after viral binding, and the relationships relating to virus transmission and population susceptibility to it. Lastly, we speculate on the pathogenesis of COVID-19 and provide a useful reference for drug development against this aggressive virus.
Keywords: 2019-nCoV; Angiotensin converting enzyme 2; Coronavirus; Immune function; Renin angiotensin system; Virus receptor.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures
References
-
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 doi: 10.1016/s0140-6736(20)30566-3. - DOI - PMC - PubMed
-
- Donoghue M, Hsieh F, Baronas E, Godbout K, Gosselin M, Stagliano N, Donovan M, Woolf B, Robison K, Jeyaseelan R, Breitbart RE, Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ Res. 2000;87(5):E1–9. doi: 10.1161/01.res.87.5.e1. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

