Glycan-Based Near-infrared Fluorescent (NIRF) Imaging of Gastrointestinal Tumors: a Preclinical Proof-of-Concept In Vivo Study
- PMID: 32780212
- PMCID: PMC7666282
- DOI: 10.1007/s11307-020-01522-8
Glycan-Based Near-infrared Fluorescent (NIRF) Imaging of Gastrointestinal Tumors: a Preclinical Proof-of-Concept In Vivo Study
Abstract
Purpose: Aberrantly expressed glycans in cancer are of particular interest for tumor targeting. This proof-of-concept in vivo study aims to validate the use of aberrant Lewis glycans as target for antibody-based, real-time imaging of gastrointestinal cancers.
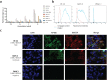
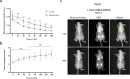
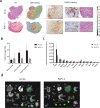
Procedures: Immunohistochemical (IHC) staining with monoclonal antibody FG88.2, targeting Lewisa/c/x, was performed on gastrointestinal tumors and their healthy counterparts. Then, FG88.2 and its chimeric human/mouse variant CH88.2 were conjugated with near-infrared fluorescent (NIRF) IRDye 800CW for real-time imaging. Specific binding was evaluated in vitro on human gastrointestinal cancer cell lines with cell-based plate assays, flow cytometry, and immune-fluorescence microscopy. Subsequently, mice bearing human colon and pancreatic subcutaneous tumors were imaged in vivo after intravenous administration of 1 nmol (150 μg) CH88.2-800CW with the clinical Artemis NIRF imaging system using the Pearl Trilogy small animal imager as reference. One week post-injection of the tracer, tumors and organs were resected and tracer uptake was analyzed ex vivo.
Results: IHC analysis showed strong FG88.2 staining on colonic, gastric, and pancreatic tumors, while staining on their normal tissue counterparts was limited. Next, human cancer cell lines HT-29 (colon) and BxPC-3 and PANC-1 (both pancreatic) were identified as respectively high, moderate, and low Lewisa/c/x-expressing. Using the clinical NIRF camera system for tumor-bearing mice, a mean tumor-to-background ratio (TBR) of 2.2 ± 0.3 (Pearl: 3.1 ± 0.8) was observed in the HT-29 tumors and a TBR of 1.8 ± 0.3 (Pearl: 1.9 ± 0.5) was achieved in the moderate expression BxPC-3 model. In both models, tumors could be adequately localized and delineated by NIRF for up to 1 week. Ex vivo analysis confirmed full tumor penetration of the tracer and low fluorescence signals in other organs.
Conclusions: Using a novel chimeric Lewisa/c/x-targeting tracer in combination with a clinical NIRF imager, we demonstrate the potential of targeting Lewis glycans for fluorescence-guided surgery of gastrointestinal tumors.
Keywords: Aberrant glycosylation; Carbohydrates; Fluorescence-guided surgery; Lewis glycans; Monoclonal antibody.
Conflict of interest statement
Lindy G. Durrant is CEO and CSO of Scancell Ltd. and has ownership interest in Scancell Ltd. Jia Xin Chua, Mireille Vankemmelbeke and Tina Parsons are employed at Scancell Ltd. All remaining authors declare that they have no conflict of interest.
Figures



Similar articles
-
Preclinical evaluation of glycan-targeting monoclonal antibodies for bimodal near-infrared fluorescence and photoacoustic imaging of gastrointestinal cancers.EJNMMI Res. 2025 Jun 6;15(1):67. doi: 10.1186/s13550-025-01258-y. EJNMMI Res. 2025. PMID: 40478321 Free PMC article.
-
In vivo near-infrared fluorescence imaging of CD105 expression during tumor angiogenesis.Eur J Nucl Med Mol Imaging. 2011 Nov;38(11):2066-76. doi: 10.1007/s00259-011-1886-x. Epub 2011 Aug 4. Eur J Nucl Med Mol Imaging. 2011. PMID: 21814852 Free PMC article.
-
Preclinical evaluation of a novel CEA-targeting near-infrared fluorescent tracer delineating colorectal and pancreatic tumors.Int J Cancer. 2015 Oct 15;137(8):1910-20. doi: 10.1002/ijc.29571. Epub 2015 Jun 22. Int J Cancer. 2015. PMID: 25895046 Free PMC article.
-
64Cu-Labeled NOTA-conjugated anti-CD105 (endoglin) chimeric monoclonal antibody linked to near-infrared dye IRDye 800CW.2012 Mar 23 [updated 2012 May 10]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Mar 23 [updated 2012 May 10]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 22593945 Free Books & Documents. Review.
-
64Cu-Labeled 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA)-conjugated IRDye 800CW (a near-infrared fluorescence dye) coupled to mAb7, an anti-epithelial cell adhesion molecule monoclonal antibody.2013 Mar 5 [updated 2013 Apr 11]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2013 Mar 5 [updated 2013 Apr 11]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 23586111 Free Books & Documents. Review.
Cited by
-
Advances in the diagnosis and treatment of MET-variant digestive tract tumors.World J Gastrointest Oncol. 2024 Nov 15;16(11):4338-4353. doi: 10.4251/wjgo.v16.i11.4338. World J Gastrointest Oncol. 2024. PMID: 39554732 Free PMC article. Review.
-
Near-Infrared Fluorescence Imaging of Pancreatic Cancer Using a Fluorescently Labelled Anti-CEA Nanobody Probe: A Preclinical Study.Biomolecules. 2023 Mar 30;13(4):618. doi: 10.3390/biom13040618. Biomolecules. 2023. PMID: 37189366 Free PMC article.
-
Application value of modified skin expansion in PICC catheterization under the guidance of B-ultrasound in gastrointestinal cancer patients with chemotherapy.Am J Transl Res. 2022 Nov 15;14(11):7932-7941. eCollection 2022. Am J Transl Res. 2022. PMID: 36505288 Free PMC article.
-
Prediction of Biomarker Expression on Primary Pancreatic Ductal Adenocarcinoma Tissues Using Fine-Needle Biopsies: Paving the Way for a Patient-Tailored Molecular Imaging Approach.Mol Diagn Ther. 2023 Mar;27(2):261-273. doi: 10.1007/s40291-022-00635-w. Epub 2023 Jan 19. Mol Diagn Ther. 2023. PMID: 36656512 Free PMC article.
-
An Immunohistochemical Evaluation of Tumor-Associated Glycans and Mucins as Targets for Molecular Imaging of Pancreatic Ductal Adenocarcinoma.Cancers (Basel). 2021 Nov 18;13(22):5777. doi: 10.3390/cancers13225777. Cancers (Basel). 2021. PMID: 34830932 Free PMC article.
References
-
- Boonstra MC, Tolner B, Schaafsma BE, Boogerd LSF, Prevoo HAJM, Bhavsar G, Kuppen PJK, Sier CFM, Bonsing BA, Frangioni JV, van de Velde CJH, Chester KA, Vahrmeijer AL. Preclinical evaluation of a novel CEA-targeting near-infrared fluorescent tracer delineating colorectal and pancreatic tumors. Int J Cancer. 2015;137:1910–1920. doi: 10.1002/ijc.29571. - DOI - PMC - PubMed
-
- van Driel PBAA, Boonstra MC, Prevoo HAJM, van de Giessen M, Snoeks TJA, Tummers QRJG, Keereweer S, Cordfunke RA, Fish A, van Eendenburg JDH, Lelieveldt BPF, Dijkstra J, van de Velde CJH, Kuppen PJK, Vahrmeijer AL, Löwik CWGM, Sier CFM. EpCAM as multi-tumour target for near-infrared fluorescence guided surgery. BMC Cancer. 2016;16:884. doi: 10.1186/s12885-016-2932-7. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

