Defibrillation testing during implantation of the subcutaneous implantable cardioverter-defibrillator: a necessary standard or becoming redundant?
- PMID: 32780342
- PMCID: PMC7419406
- DOI: 10.1007/s12471-020-01448-4
Defibrillation testing during implantation of the subcutaneous implantable cardioverter-defibrillator: a necessary standard or becoming redundant?
Abstract
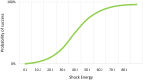
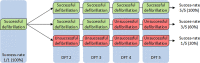
Since the publication of the SIMPLE and NORDIC trials, defibrillation testing (DFT) is rarely performed during routine implantation of transvenous implantable cardioverter-defibrillators (ICD). However, the results of these trials cannot be extrapolated to the later introduced subcutaneous ICD (S-ICD) and a class I recommendation to perform DFT during the implantation of these devices remains in the current guidelines. Due to the high conversion success rate of DFT on one hand, and the risk of complications on the other, a significant number of physicians omit DFT in S‑ICD recipients. Several retrospective analyses have assessed the safety of the omission of DFT and report contradicting results and recommendations. It is known that implant position, as well as device factors and patient characteristics, influence defibrillation success. A better comprehension of these factors and their relationship could lead to more reliable and safer alternatives to DFT. An ongoing randomised clinical trial, which is expected to end in 2023, is the first study to implement a method that assesses implant position to identify patients who are likely to fail their DFT.
Keywords: Defibrillation testing; Implantable defibrillator; Safety margin testing; Sudden cardiac death; Ventricular fibrillation.
Conflict of interest statement
W. van der Stuijt and A.B.E. Quast have nothing to disclose. R.E. Knops reports consultancy fees and research grants from Abbott, Boston Scientific, Cairdac and Medtronic, as well as stock options from AtaCor Medical Inc.
Figures
References
-
- Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15:e73–e189. doi: 10.1016/j.hrthm.2017.10.036. - DOI - PubMed
-
- Priori SG, Blomstrom-Lundqvist C, Mazzanti A, et al. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC) Europace. 2015;17:1601–1687. - PubMed
Publication types
LinkOut - more resources
Full Text Sources