Comparison analysis of different swabs and transport mediums suitable for SARS-CoV-2 testing following shortages
- PMID: 32781008
- PMCID: PMC7414358
- DOI: 10.1016/j.jviromet.2020.113947
Comparison analysis of different swabs and transport mediums suitable for SARS-CoV-2 testing following shortages
Abstract
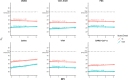
On March 11, 2020, the World Health Organization (WHO) assessed COVID-19, caused by SARS-CoV-2, as a pandemic. As of June 1, 2020, SARS-CoV-2 has had a documented effect of over 6 million cases world-wide, amounting to over 370,000 deaths (World Health Organization, 2020. Novel Coronavirus (COVID-19) Situation. http://https://covid19.who.int/). Consequently, the high demand for testing has resulted in a depletion of commercially available consumables, including the recommended swabs and viral transport media (VTM) required for nasopharyngeal sampling. Therefore, the potential use of unvalidated alternatives must be explored to address the global shortage of testing supplies. To tackle this issue, we evaluated the utility of different swabs and transport mediums for the molecular detection of SARS-CoV-2. This study compared the performance of six swabs commonly found in primary and tertiary health care settings (PurFlock Ultra, FLOQSwab, Puritan Pur-Wraps cotton tipped applicators, Puritan polyester tipped applicators, MedPro 6" cotton tipped applicators, and HOLOGIC Aptima) for their efficacy in testing for SARS-CoV-2. Separately, the molecular detection of SARS-CoV-2 was completed from different transport mediums (DMEM, PBS, 100 % ethanol, 0.9 % normal saline and VTM), which were kept up to three days at room temperature (RT). The results indicate that there is no meaningful difference in viral yield from different swabs and most transport mediums for the collection and detection of SARS-CoV-2, indicating swab and medium alternatives could be used if supplies run out.
Keywords: Diagnostic testing; SARS-CoV-2; Swab.
Copyright © 2020. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
-
- Centre for Disease Control and Prevention . 2018. Collecting Specimens for Varicella Zoster Virus (VZV) Testing.
-
- Centre for Disease Control and Prevention . 2020. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19) [WWW Document] URL https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specim... (Accessed 3.24.20)
-
- Copan Diagnostics . 2020. FLOQSwab [WWW Document]
-
- Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K., Bleicker T., Brünink S., Schneider J., Schmidt M.L., Mulders D.G., Haagmans B.L., van der Veer B., van den Brink S., Wijsman L., Goderski G., Romette J.L., Ellis J., Zambon M., Peiris M., Goossens H., Reusken C., Koopmans M.P., Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:1–8. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

