Poly(ε-caprolactone) Titanium Dioxide and Cefuroxime Antimicrobial Scaffolds for Cultivation of Human Limbal Stem Cells
- PMID: 32781567
- PMCID: PMC7465675
- DOI: 10.3390/polym12081758
Poly(ε-caprolactone) Titanium Dioxide and Cefuroxime Antimicrobial Scaffolds for Cultivation of Human Limbal Stem Cells
Abstract
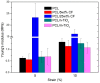
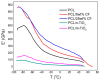
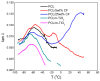
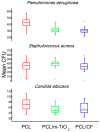
Limbal Stem Cell Deficiency (LSCD) is a very serious and painful disease that often results in impaired vision. Cultivation of limbal stem cells for clinical application is usually performed on carriers such as amniotic membrane or surgical fibrin gel. Transplantation of these grafts is associated with the risk of local postoperative infection that can destroy the graft and devoid therapeutic benefit. For this reason, electrospun scaffolds are good alternatives, as proven to mimic the natural cells surroundings, while their fabrication technique is versatile with regard to polymer functionalization and scaffolds architecture. This study considers the development of poly(ε-caprolactone) (PCL) immune-compatible and biodegradable electrospun scaffolds, comprising cefuroxime (CF) or titanium dioxide (TiO2) active components, that provide both bactericidal activity against eye infections and support of limbal stem cells growth in vitro. The PCL/CF scaffolds were prepared by blend electrospinning, while functionalization with the TiO2 particles was performed by ultrasonic post-processing treatment. The fabricated scaffolds were evaluated in regard to their physical structure, wetting ability, static and dynamic mechanical behaviour, antimicrobial efficiency and drug release, through scanning electron microscopy, water contact angle measurement, tensile testing and dynamic mechanical analysis, antimicrobial tests and UV-Vis spectroscopy, respectively. Human limbal stem cells, isolated from surgical remains of human cadaveric cornea, were cultured on the PCL/CF and PCL/TiO2 scaffolds and further identified through immunocytochemistry in terms of cell type thus were stained against p63 marker for limbal stem cells, a nuclear transcription factor and cytokeratin 3 (CK3), a corneal epithelial differentiation marker. The electrospun PCL/CF and PCL/TiO2 successfully supported the adhesion, proliferation and differentiation of the cultivated limbal cells and provided the antimicrobial effect against Pseudomonas aeruginosa, Staphylococcus aureus and Candida albicans.
Keywords: antimicrobial activity; cefuroxime; electrospinning; limbal stem cell deficiency; polycaprolactone; scaffolds; tissue engineering; titanium dioxide.
Conflict of interest statement
The authors declare no conflict of interest.
Figures














References
-
- Zafar M.S., Khurshid Z., Almas K. Oral tissue engineering progress and challenges. Tissue Eng. Regen. Med. 2015;12:387–397. doi: 10.1007/s13770-015-0030-6. - DOI
-
- Castells-Sala C., Alemany-Ribes M., Fernández-Muiños T., Recha-Sancho L., López-Chicón P., Aloy-Reverté C., Caballero-Camino J., Márquez-Gil A., Semino C.E. Current applications of tissue engineering in biomedicine. J. Biochips. Tiss. Chip. 2013;1 doi: 10.4172/2153-0777. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

