Plasticity of the 340-Loop in Influenza Neuraminidase Offers New Insight for Antiviral Drug Development
- PMID: 32781779
- PMCID: PMC7460844
- DOI: 10.3390/ijms21165655
Plasticity of the 340-Loop in Influenza Neuraminidase Offers New Insight for Antiviral Drug Development
Abstract
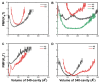
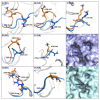
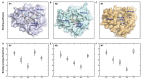
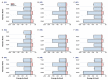
The recently discovered 340-cavity in influenza neuraminidase (NA) N6 and N7 subtypes has introduced new possibilities for rational structure-based drug design. However, the plasticity of the 340-loop (residues 342-347) and the role of the 340-loop in NA activity and substrate binding have not been deeply exploited. Here, we investigate the mechanism of 340-cavity formation and demonstrate for the first time that seven of nine NA subtypes are able to adopt an open 340-cavity over 1.8 μs total molecular dynamics simulation time. The finding that the 340-loop plays a role in the sialic acid binding pathway suggests that the 340-cavity can function as a druggable pocket. Comparing the open and closed conformations of the 340-loop, the side chain orientation of residue 344 was found to govern the formation of the 340-cavity. Additionally, the conserved calcium ion was found to substantially influence the stability of the 340-loop. Our study provides dynamical evidence supporting the 340-cavity as a druggable hotspot at the atomic level and offers new structural insight in designing antiviral drugs.
Keywords: 340-cavity; influenza; molecular dynamics simulations; neuraminidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

