Silicon-silicon π single bond
- PMID: 32782244
- PMCID: PMC7419521
- DOI: 10.1038/s41467-020-17815-z
Silicon-silicon π single bond
Abstract
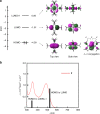
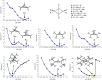
A carbon-carbon double bond consists of a σ bond and a π bond. Recently, the concept of a π single bond, where a π bond is not accompanied by a σ bond, has been proposed in diradicals containing carbon and heteroatom radical centers. Here we report a closed-shell compound having a silicon-silicon π single bond. 1,2,2,3,4,4-Hexa-tert-butylbicyclo[1.1.0]tetrasilane has a silicon-silicon π single bond between the bridgehead silicon atoms. The X-ray crystallographic analysis shows that the silicon-silicon π single bond (2.853(1) Å) is far longer than the longest silicon-silicon bond so far reported. In spite of this unusually long bond length, the electrons of the 3p orbitals are paired, which is confirmed by measurement of electron paramagnetic resonance, and magnetic susceptibility and natural bond orbital analysis. The properties of the silicon-silicon π single bond are studied by UV/Vis and 29Si NMR spectroscopy, and theoretical calculations.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Nakagaki T, Sakai T, Mizuta T, Fujiwara Y, Abe M. Kinetic stabilization and reactivity of π single-bonded species: effect of the alkoxy group on the lifetime of singlet 2,2-dialkoxy-1,3-diphenyloctahydropentalene-1,3-diyls. Chem. Eur. J. 2013;19:10395–10404. - PubMed
-
- Abe M, Akisaka R. Is π–single bonding (C–π–C) possible? A challenge in organic chemistry. Chem. Lett. 2017;46:1586–1592.
-
- Akisaka R, Abe M. Bulky substituent effect on reactivity of localized singlet cyclopentane-1,3-diyls with π–single bonding (C–π–C) character. Chem. Asian J. 2019;14:4223–4228. - PubMed
-
- Breher F. Stretching bonds in main group element compounds—borderlines between biradicals and closed-shell species. Coord. Chem. Rev. 2007;251:1007–1043.
-
- Abe M. Diradicals. Chem. Rev. 2013;113:7011–7088. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

