Advances in oligonucleotide drug delivery
- PMID: 32782413
- PMCID: PMC7419031
- DOI: 10.1038/s41573-020-0075-7
Advances in oligonucleotide drug delivery
Abstract
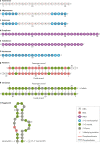
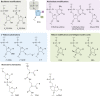
Oligonucleotides can be used to modulate gene expression via a range of processes including RNAi, target degradation by RNase H-mediated cleavage, splicing modulation, non-coding RNA inhibition, gene activation and programmed gene editing. As such, these molecules have potential therapeutic applications for myriad indications, with several oligonucleotide drugs recently gaining approval. However, despite recent technological advances, achieving efficient oligonucleotide delivery, particularly to extrahepatic tissues, remains a major translational limitation. Here, we provide an overview of oligonucleotide-based drug platforms, focusing on key approaches - including chemical modification, bioconjugation and the use of nanocarriers - which aim to address the delivery challenge.
Conflict of interest statement
Complete details of relationships, compensated and uncompensated, for R.L. can be found in the Supplementary information. M.J.A.W. is a founder and shareholder of Evox Therapeutics and PepGen Ltd, companies dedicated to the commercialization of extracellular vesicle therapeutics and peptide-enhanced therapeutic oligonucleotide delivery, respectively. T.C.R. declares no competing financial interests.
Figures


References
-
- Giorgio E, et al. Allele-specific silencing as treatment for gene duplication disorders: proof-of-principle in autosomal dominant leukodystrophy. Brain. 2019;142:1905–1920. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

