Cervical cancer in low and middle-income countries
- PMID: 32782524
- PMCID: PMC7400218
- DOI: 10.3892/ol.2020.11754
Cervical cancer in low and middle-income countries
Abstract
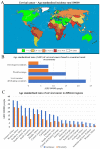
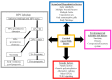
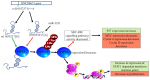
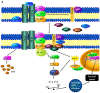
Cervical cancer is a malignant tumour that occurs in the cervix and is classified into two histological types, adenocarcinoma and squamous cell carcinoma (SCC); SCC is more common and accounts for 70% of all cases. In 2018 there were ~569,000 new cases of cervical cancer diagnosed worldwide and ~311,000 deaths were attributed to cervical cancer. Of these, between 84 and 90% occurred in low- and middle-income countries (LMICs) such as South Africa, India, China and Brazil. The most common cause of cervical cancer is persistent infection caused by the sexually transmitted human papilloma virus. Other factors that contribute to the incidence of cervical cancer include geography, traditional practices and beliefs, the screening levels, socioeconomic status, healthcare access, public awareness, use of oral contraceptives, smoking and co-infection with HIV. An estimated 11 million women from LMICs will be diagnosed with cervical cancer in the next 10-20 years. The aim of this review was to explore various types of genetic and epigenetic factors that influence the development, progression or suppression of cervical cancer.
Keywords: Brazil; India; South Africa; Tanzania; cervical cancer; miRNA.
Copyright: © Hull et al.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Medical
Research Materials
