The combination of orlistat, lonidamine and 6-diazo-5-oxo-L-norleucine induces a quiescent energetic phenotype and limits substrate flexibility in colon cancer cells
- PMID: 32782623
- PMCID: PMC7400019
- DOI: 10.3892/ol.2020.11838
The combination of orlistat, lonidamine and 6-diazo-5-oxo-L-norleucine induces a quiescent energetic phenotype and limits substrate flexibility in colon cancer cells
Abstract
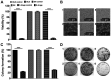
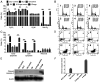
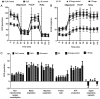
Cancer upregulates glycolysis, glutaminolysis and lipogenesis, and induces a catabolic state in patients. The concurrent inhibition of both tumor anabolism and host catabolism, and the energetic consequences of such an approach, have not previously been fully investigated. In the present study, CT26.WT murine colon cancer cells were treated with the combination of anti-anabolic drugs orlistat, lonidamine and 6-diazo-5-oxo-L-norleucine (DON; OLD scheme), which are inhibitors of the de novo synthesis of fatty acids, glycolysis and glutaminolysis, respectively. In addition, the effects of OLD scheme sumplemented with the combination of anti-catabolic compounds, namely growth hormone, insulin and indomethacin (GII scheme), were also evaluated. The effects of the compounds used in combination on CT26.WT cell viability, clonogenicity and energetic metabolism were assessed in vitro. The results demonstrated that the anti-anabolic approach reduced cell viability, clonogenicity and cell cycle progression, and increased apoptosis. These effects were associated with decreased oxidative phosphorylation, glycolysis and fuel flexibility. Furthermore, the anti-catabolic scheme, alone or supplemented with anti-anabolic compounds, did not favor tumor growth. These findings indicated that the simultaneous pharmacological inhibition of tumor anabolism and host catabolism exhibits antitumor effects that should be further evaluated.
Keywords: colon cancer; de novo fatty acid synthesis; glutaminolysis; glycolysis; metabolism.
Copyright © 2020, Spandidos Publications.
Figures
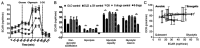

References
LinkOut - more resources
Full Text Sources
