Are anti-PD1 and anti-PD-L1 alike? The non-small-cell lung cancer paradigm
- PMID: 32782728
- PMCID: PMC7385529
- DOI: 10.4081/oncol.2020.490
Are anti-PD1 and anti-PD-L1 alike? The non-small-cell lung cancer paradigm
Abstract
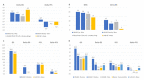
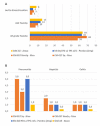
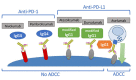
Anti-PD1 and anti-PD-L1 agents may have intrinsic and clinically relevant differences in the treatment of non-small cell lung cancer (NSCLC) patients. By reviewing currently available indirect evidence on these agents for NSCLC treatment, highlighting possible inter- and intra-class dissimilarities, anti-PD1 agents showed a higher response rate and a better outcome when combined with chemotherapy for the first-line treatment of patients with squamous and PD-L1 low advanced NSCLC, as compared to anti-PD-L1 agents. Conversely, anti-PD-L1 agents were responsible for less severe adverse events (AEs), particularly, immunerelated AEs. These differences could be explained by their different specific properties. Considering possible differences between anti-PD1 and anti-PD-L1 agents could be clinically relevant for treatment tailoring and inspiring new investigational approaches.
Keywords: Immune-checkpoint inhibitor; PD-L1; PD1; immune-related adverse events; lung cancer.
©Copyright: the Author(s).
Figures
References
-
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. - PubMed
-
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019;393:1819-30. - PubMed
-
- Reinmuth N, Cho BC, Lee KH, et al. LBA4Effect of poststudy immunotherapy (IO) on overall survival (OS) outcome in patients with metastatic (m) NSCLC treated with first-line durvalumab (D) vs chemotherapy (CT) in the phase III MYSTIC study. Ann Oncol 2019;30.
-
- Spigel D, de Marinis F, Giaccone G, et al. LBA78IMpower110: Interim overall survival (OS) analysis of a phase III study of atezolizumab (atezo) vs platinum-based chemotherapy (chemo) as first-line (1L) treatment (tx) in PDL1– selected NSCLC. Ann Oncol 2019;30.
LinkOut - more resources
Full Text Sources
Research Materials

