Graphene Oxide Induces Ester Bonds Hydrolysis of Poly-l-lactic Acid Scaffold to Accelerate Degradation
- PMID: 32782986
- PMCID: PMC7415862
- DOI: 10.18063/ijb.v6i1.249
Graphene Oxide Induces Ester Bonds Hydrolysis of Poly-l-lactic Acid Scaffold to Accelerate Degradation
Erratum in
-
ERRATUM.Int J Bioprint. 2020 Sep 17;6(4):309. doi: 10.18063/ijb.v6i4.309. eCollection 2020. Int J Bioprint. 2020. PMID: 33102924 Free PMC article.
Abstract
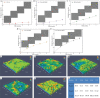
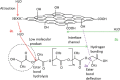
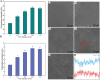
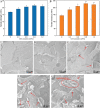
Poly-l-lactic acid (PLLA) possesses good biocompatibility and bioabsorbability as scaffold material, while slow degradation rate limits its application in bone tissue engineering. In this study, graphene oxide (GO) was introduced into the PLLA scaffold prepared by selective laser sintering to accelerate degradation. The reason was that GO with a large number of oxygen-containing functional groups attracted water molecules and transported them into scaffold through the interface microchannels formed between lamellar GO and PLLA matrix. More importantly, hydrogen bonding interaction between the functional groups of GO and the ester bonds of PLLA induced the ester bonds to deflect toward the interfaces, making water molecules attack the ester bonds and thereby breaking the molecular chain of PLLA to accelerate degradation. As a result, some micropores appeared on the surface of the PLLA scaffold, and mass loss was increased from 0.81% to 4.22% after immersing for 4 weeks when 0.9% GO was introduced. Besides, the tensile strength and compressive strength of the scaffolds increased by 24.3% and 137.4%, respectively, due to the reinforced effect of GO. In addition, the scaffold also demonstrated good bioactivity and cytocompatibility.
Keywords: Degradation property; Ester bonds hydrolysis; GO; Poly-l-lactic acid scaffold.
Copyright: © 2020 Shuai, et al.
Figures



References
-
- An J, Teoh JE, Suntornnond R, et al. Design and 3D Printing of Scaffolds and Tissues. Engineering. 2015;1:261–268.
-
- Cardoso GB, Perea GN, D'Avila MA, et al. Initial Study of Electrospinning PCL/PLLA Blends. Adv Mater Phys Chem. 2011;1:94–98.
-
- Saito Y, Minami K, Kobayashi M, et al. New Tubular Bioabsorbable Knitted Airway Stent:Biocompatibility and Mechanical Strength. J Thorac Cardiovasc Surg. 2002;123:161–167. DOI:10.1067/mtc.2002.118503. - PubMed
-
- Shuai C, Yang W, He C, et al. A Magnetic Micro-environment in Scaffolds for Stimulating Bone Regeneration. Mater Des. 2020;185(108275) DOI:10.1016/j.matdes.2019.108275.
LinkOut - more resources
Full Text Sources
Research Materials
