3D-printed Biomimetic Bioactive Glass Scaffolds for Bone Regeneration in Rat Calvarial Defects
- PMID: 32782995
- PMCID: PMC7415861
- DOI: 10.18063/ijb.v6i2.274
3D-printed Biomimetic Bioactive Glass Scaffolds for Bone Regeneration in Rat Calvarial Defects
Erratum in
-
ERRATUM.Int J Bioprint. 2020 Sep 17;6(4):309. doi: 10.18063/ijb.v6i4.309. eCollection 2020. Int J Bioprint. 2020. PMID: 33102924 Free PMC article.
Abstract
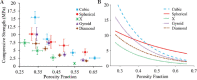
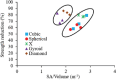
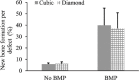
The pore geometry of scaffold intended for the use in the bone repair or replacement is one of the most important parameters in bone tissue engineering. It affects not only the mechanical properties of the scaffold but also the amount of bone regeneration after implantation. Scaffolds with five different architectures (cubic, spherical, x, gyroid, and diamond) at different porosities were fabricated with bioactive borate glass using the selective laser sintering (SLS) process. The compressive strength of scaffolds with porosities ranging from 60% to 30% varied from 1.7 to 15.5 MPa. The scaffold's compressive strength decreased significantly (up to 90%) after 1-week immersion in simulated body fluids. Degradation of scaffolds is dependent on porosity, in which the scaffold with the largest surface area has the largest reduction in strength. Scaffolds with traditional cubic architecture and biomimetic diamond architecture were implanted in 4.6 mm diameter full-thickness rat calvarial defects for 6 weeks to evaluate the bone regeneration with or without bone morphogenetic protein 2 (BMP-2). Histological analysis indicated no significant difference in bone formation in the defects treated with the two different architectures. However, the defects treated with the diamond architecture scaffolds had more fibrous tissue formation and thus have the potential for faster bone formation. Overall, the results indicated that borate glass scaffolds fabricated using the SLS process have the potential for bone repair and the addition of BMP-2 significantly improves bone regeneration.
Keywords: Bioactive borate glass; In vivo bone formation; Pore geometry; Porosity; Scaffold architecture; Selective laser sintering.
Copyright: © 2020 Kolan, et al.
Figures






References
-
- Jones JR. Review of Bioactive Glass:From Hench to Hybrids. Acta Biomater. 2013;9:4457–86. - PubMed
-
- Hench LL. The Story of Bioglass®. J Mater Sci Mater Med. 2006;17:967–78. - PubMed
-
- Greenspan D. Bioglass at 50-A Look at Larry Hench's Legacy and Bioactive Materials. Biomed Glas. 2019;5:178–84. DOI:10.1515/bglass-2019-0014.
-
- Fu Q, Rahaman MN, Fu H, et al. Silicate, Borosilicate, and Borate Bioactive Glass Scaffolds with Controllable Degradation Rate for Bone Tissue Engineering Applications. I. Preparation and In Vitro. Degradation J Biomed Mater Res Part A. 2010;95A:164–71. DOI:10.1002/jbm.a.32824. - PubMed
LinkOut - more resources
Full Text Sources
