Inhibition of Aquaporin 4 Decreases Amyloid Aβ40 Drainage Around Cerebral Vessels
- PMID: 32783141
- PMCID: PMC7515968
- DOI: 10.1007/s12035-020-02044-8
Inhibition of Aquaporin 4 Decreases Amyloid Aβ40 Drainage Around Cerebral Vessels
Abstract
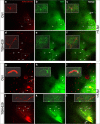
Aquaporin-4 (AQP4) is located mainly in the astrocytic end-feet around cerebral blood vessels and regulates ion and water homeostasis in the brain. While deletion of AQP4 is shown to reduce amyloid-β (Aβ) clearance and exacerbate Aβ peptide accumulation in plaques and vessels of Alzheimer's disease mouse models, the mechanism and clearing pathways involved are debated. Here, we investigated how inhibiting the function of AQP4 in healthy male C57BL/6 J mice impacts clearance of Aβ40, the more soluble Aβ isoform. Using two-photon in vivo imaging and visualizing vessels with Sulfurodamine 101 (SR101), we first showed that Aβ40 injected as a ≤ 0.5-μl volume in the cerebral cortex diffused rapidly in parenchyma and accumulated around blood vessels. In animals treated with the AQP4 inhibitor TGN-020, the perivascular Aβ40 accumulation was significantly (P < 0.001) intensified by involving four times more vessels, thus suggesting a generalized clearance defect associated with vessels. Increasing the injecting volume to ≥ 0.5 ≤ 1 μl decreased the difference of Aβ40-positive vessels observed in non-treated and AQP4 inhibitor-treated animals, although the difference was still significant (P = 0.001), suggesting that larger injection volumes could overwhelm intramural vascular clearance mechanisms. While both small and large vessels accumulated Aβ40, for the ≤ 0.5-μl volume group, the average diameter of the Aβ40-positive vessels tended to be larger in control animals compared with TGN-020-treated animals, although the difference was non-significant (P = 0.066). Using histopathology and ultrastructural microscopy, no vascular structural change was observed after a single massive dose of TGN-020. These data suggest that AQP4 deficiency is directly involved in impaired Aβ brain clearance via the peri-/para-vascular routes, and AQP4-mediated vascular clearance might counteract blood-brain barrier abnormalities and age-related vascular amyloidopathy.
Keywords: Alzheimer’s disease; Amyloid; Aquaporin 4; Aβ40; Perivascular drainage; TGN-020.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

