Tapering Canakinumab Monotherapy in Patients With Systemic Juvenile Idiopathic Arthritis in Clinical Remission: Results From a Phase IIIb/IV Open-Label, Randomized Study
- PMID: 32783351
- PMCID: PMC7898684
- DOI: 10.1002/art.41488
Tapering Canakinumab Monotherapy in Patients With Systemic Juvenile Idiopathic Arthritis in Clinical Remission: Results From a Phase IIIb/IV Open-Label, Randomized Study
Abstract
Objective: To evaluate the efficacy and safety of 2 canakinumab monotherapy tapering regimens in order to maintain complete clinical remission in children with systemic juvenile idiopathic arthritis (JIA).
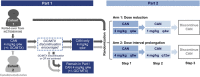
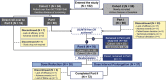
Methods: The study was designed as a 2-part phase IIIb/IV open-label, randomized trial. In the first part, patients received 4 mg/kg of canakinumab subcutaneously every 4 weeks and discontinued glucocorticoids and/or methotrexate as appropriate. Patients in whom clinical remission was achieved (inactive disease for at least 24 weeks) with canakinumab monotherapy were entered into the second part of the trial, in which they were randomized 1:1 into 1 of 2 treatment arms. In arm 1, the dose of canakinumab was reduced from 4 mg/kg to 2 mg/kg and then to 1 mg/kg, followed by discontinuation. In arm 2, the 4 mg/kg dose interval was prolonged from every 4 weeks, to every 8 weeks, and then to every 12 weeks, followed by discontinuation. In both arms, canakinumab exposure could be reduced provided systemic JIA remained in clinical remission for 24 weeks with each step. The primary objective was to assess whether >40% of randomized patients in either arm maintained clinical remission of systemic JIA for 24 weeks in the first part of the study.
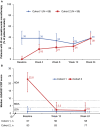
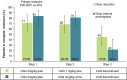
Results: In part 1 of the study, 182 patients were enrolled, with 75 of those patients randomized before entering part 2 of the trial. Among the 75 randomized patients, clinical remission was maintained for 24 weeks in 27 (71%) of 38 patients in arm 1 (2 mg/kg every 4 weeks) and 31 (84%) of 37 patients in arm 2 (4 mg/kg every 8 weeks) (P ≤ 0.0001 for arm 1 versus arm 2 among those meeting the 40% threshold). Overall, 25 (33%) of 75 patients discontinued canakinumab, and clinical remission was maintained for at least 24 weeks in all 25 of these patients. No new safety signals were identified.
Conclusion: Reduction of canakinumab exposure may be feasible in patients who have achieved clinical remission of systemic JIA, but consistent interleukin-1 inhibition appears necessary to maintain this response.
Trial registration: ClinicalTrials.gov NCT02296424.
© 2020 The Authors. Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures
References
-
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol 2004;31:390–2. - PubMed
-
- Prakken B, Albani S, Martini A. Juvenile idiopathic arthritis. Lancet 2011;377:2138–49. - PubMed
-
- Ravelli A, Martini A. Juvenile idiopathic arthritis. Lancet 2007;369:767–78. - PubMed
-
- Martini A, Ravelli A, di Fuccia G, Rosti V, Cazzola M, Barosi G. Intravenous iron therapy for severe anaemia in systemic‐onset juvenile chronic arthritis. Lancet 1994;344:1052–4. - PubMed
-
- Consolaro A, Giancane G, Alongi A, van Dijkhuizen EH, Aggarwal A, al‐Mayouf SM, et al. Phenotypic variability and disparities in treatment and outcomes of childhood arthritis throughout the world: an observational cohort study. Lancet Child Adolesc Heal 2019;3:255–63. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

