Structural Basis for Helicase-Polymerase Coupling in the SARS-CoV-2 Replication-Transcription Complex
- PMID: 32783916
- PMCID: PMC7386476
- DOI: 10.1016/j.cell.2020.07.033
Structural Basis for Helicase-Polymerase Coupling in the SARS-CoV-2 Replication-Transcription Complex
Abstract
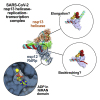
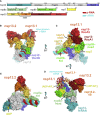
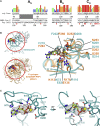
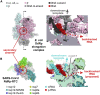
SARS-CoV-2 is the causative agent of the 2019-2020 pandemic. The SARS-CoV-2 genome is replicated and transcribed by the RNA-dependent RNA polymerase holoenzyme (subunits nsp7/nsp82/nsp12) along with a cast of accessory factors. One of these factors is the nsp13 helicase. Both the holo-RdRp and nsp13 are essential for viral replication and are targets for treating the disease COVID-19. Here we present cryoelectron microscopic structures of the SARS-CoV-2 holo-RdRp with an RNA template product in complex with two molecules of the nsp13 helicase. The Nidovirales order-specific N-terminal domains of each nsp13 interact with the N-terminal extension of each copy of nsp8. One nsp13 also contacts the nsp12 thumb. The structure places the nucleic acid-binding ATPase domains of the helicase directly in front of the replicating-transcribing holo-RdRp, constraining models for nsp13 function. We also observe ADP-Mg2+ bound in the nsp12 N-terminal nidovirus RdRp-associated nucleotidyltransferase domain, detailing a new pocket for anti-viral therapy development.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures



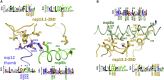

Update of
-
Structural basis for helicase-polymerase coupling in the SARS-CoV-2 replication-transcription complex.bioRxiv [Preprint]. 2020 Jul 13:2020.07.08.194084. doi: 10.1101/2020.07.08.194084. bioRxiv. 2020. Update in: Cell. 2020 Sep 17;182(6):1560-1573.e13. doi: 10.1016/j.cell.2020.07.033. PMID: 32676607 Free PMC article. Updated. Preprint.
-
Structural basis for backtracking by the SARS-CoV-2 replication-transcription complex.bioRxiv [Preprint]. 2021 Mar 14:2021.03.13.435256. doi: 10.1101/2021.03.13.435256. bioRxiv. 2021. Update in: Cell. 2020 Sep 17;182(6):1560-1573.e13. doi: 10.1016/j.cell.2020.07.033. Update in: Proc Natl Acad Sci U S A. 2021 May 11;118(19):e2102516118. doi: 10.1073/pnas.2102516118. PMID: 33758867 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

