Recruiting Adolescents With Chronic Fatigue Syndrome/Myalgic Encephalomyelitis to Internet-Delivered Therapy: Internal Pilot Within a Randomized Controlled Trial
- PMID: 32784188
- PMCID: PMC7450376
- DOI: 10.2196/17768
Recruiting Adolescents With Chronic Fatigue Syndrome/Myalgic Encephalomyelitis to Internet-Delivered Therapy: Internal Pilot Within a Randomized Controlled Trial
Abstract
Background: Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) in adolescents is common and disabling. Teenagers in the United Kingdom are more likely to recover if they access specialist care, but most do not have access to a local specialist CFS/ME service. Delivering treatment remotely via the internet could improve access to treatment.
Objective: This study aims to assess (1) the feasibility of recruitment and retention into a trial of internet-delivered specialist treatment for adolescents with CFS/ME and (2) the acceptability of trial processes and 2 web-based treatments (to inform continuation to full trial).
Methods: This study is an internal pilot for the initial 12 months of a full randomized controlled trial (RCT), with integrated qualitative methods (analysis of recruitment consultations and participant and clinician interviews). Recruitment and treatment were delivered remotely from a specialist pediatric CFS/ME treatment service within a hospital in South West United Kingdom. Adolescents (aged 11-17 years) from across the United Kingdom with a diagnosis of CFS/ME and no access to local specialist treatment were referred by their general practitioner to the treatment center. Eligibility assessment and recruitment were conducted via remote methods (telephone and on the web), and participants were randomized (via a computer-automated system) to 1 of 2 web-based treatments. The trial intervention was Fatigue in Teenagers on the InterNET in the National Health Service, a web-based modular CFS/ME-specific cognitive behavioral therapy program (designed to be used by young people and their parents or caregivers) supported by individualized clinical psychologist electronic consultations (regular, scheduled therapeutic message exchanges between participants and therapist within the platform). The comparator was Skype-delivered activity management with a CFS/ME clinician (mainly a physiotherapist or occupational therapist). Both treatments were intended to last for up to 6 months. The primary outcomes were (1) the number of participants recruited (per out-of-area referrals received between November 1, 2016, to October 31, 2017) and the proportion providing 6-month outcome data (web-based self-report questionnaire assessing functioning) and (2) the qualitative outcomes indicating the acceptability of trial processes and treatments.
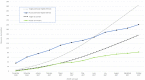
Results: A total of 89 out of 150 (59.3% of potentially eligible referrals) young people and their parents or caregivers were recruited, with 75 out of 89 (84.2%) providing 6-month outcome data. Overall, web-based treatment was acceptable; however, participants and clinicians described both the advantages and disadvantages of remote methods. No serious adverse events were reported.
Conclusions: Recruiting young people (and their parents or caregivers) into an RCT of web-based treatment via remote methods is feasible and acceptable. Delivering specialist treatment at home via the internet is feasible and acceptable, although some families prefer to travel across the United Kingdom for face-to-face treatment.
Trial registration: ISRCTN 18020851; http://www.isrctn.com/ISRCTN18020851.
International registered report identifier (irrid): RR2-10.1186/s13063-018-2500-3.
Keywords: chronic fatigue syndrome; cognitive behavioral therapy; e-counseling; e-therapy; eHealth; myalgic encephalomyelitis; online systems; pediatrics; pilot projects; qualitative research.
©Emma Anderson, Roxanne Parslow, William Hollingworth, Nicola Mills, Lucy Beasant, Daisy Gaunt, Chris Metcalfe, David Kessler, John Macleod, Susan Pywell, Kieren Pitts, Simon Price, Paul Stallard, Hans Knoop, Elise Van de Putte, Sanne Nijhof, Gijs Bleijenberg, Esther Crawley. Originally published in the Journal of Medical Internet Research (http://www.jmir.org), 12.08.2020.
Conflict of interest statement
Conflicts of Interest: HK and GB received royalties for the publication of a treatment manual for CBT in CFS/ME in adults. The remaining authors have no conflicts of interest to disclose.
Figures
References
-
- Crawley EM, Emond AM, Sterne JA. Unidentified chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is a major cause of school absence: surveillance outcomes from school-based clinics. BMJ Open. 2011;1(2):e000252. doi: 10.1136/bmjopen-2011-000252. http://bmjopen.bmj.com/cgi/pmidlookup?view=long&pmid=22155938 - DOI - PMC - PubMed
-
- Rangel L, Garralda ME, Levin M, Roberts H. The course of severe chronic fatigue syndrome in childhood. J R Soc Med. 2000 Mar;93(3):129–34. doi: 10.1177/014107680009300306. http://europepmc.org/abstract/MED/10741312 - DOI - PMC - PubMed
-
- Nijhof SL, Bleijenberg G, Uiterwaal CS, Kimpen JL, van de Putte EM. Effectiveness of internet-based cognitive behavioural treatment for adolescents with chronic fatigue syndrome (FITNET): a randomised controlled trial. Lancet. 2012 Apr 14;379(9824):1412–8. doi: 10.1016/S0140-6736(12)60025-7. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

