Mapping Attenuation Determinants in Enterovirus-D68
- PMID: 32784424
- PMCID: PMC7472100
- DOI: 10.3390/v12080867
Mapping Attenuation Determinants in Enterovirus-D68
Abstract
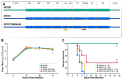
Enterovirus (EV)-D68 has been associated with epidemics in the United Sates in 2014, 2016 and 2018. This study aims to identify potential viral virulence determinants. We found that neonatal type I interferon receptor knockout mice are susceptible to EV-D68 infection via intraperitoneal inoculation and were able to recapitulate the paralysis process observed in human disease. Among the EV-D68 strains tested, strain US/MO-14-18949 caused no observable disease in this mouse model, whereas the other strains caused paralysis and death. Sequence analysis revealed several conserved genetic changes among these virus strains: nucleotide positions 107 and 648 in the 5'-untranslated region (UTR); amino acid position 88 in VP3; 1, 148, 282 and 283 in VP1; 22 in 2A; 47 in 3A. A series of chimeric and point-mutated infectious clones were constructed to identify viral elements responsible for the distinct virulence. A single amino acid change from isoleucine to valine at position 88 in VP3 attenuated neurovirulence by reducing virus replication in the brain and spinal cord of infected mice.
Keywords: VP3; enterovirus; enterovirus-D68; infectious clones; mouse model; paralysis; virulence determinant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Meijer A., Van der Sanden S., Snijders B.E., Jaramillo-Gutierrez G., Bont L., Van der Ent C.K., Overduin P., Jenny S.L., Jusic E., Van der Avoort H.G., et al. Emergence and epidemic occurrence of enterovirus 68 respiratory infections in The Netherlands in 2010. Virology. 2012;423:49–57. doi: 10.1016/j.virol.2011.11.021. - DOI - PubMed

