Metabolic Dysregulation in Idiopathic Pulmonary Fibrosis
- PMID: 32784632
- PMCID: PMC7461042
- DOI: 10.3390/ijms21165663
Metabolic Dysregulation in Idiopathic Pulmonary Fibrosis
Abstract
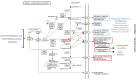
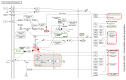
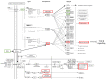
Idiopathic pulmonary fibrosis (IPF) is a fibroproliferative disorder limited to the lung. New findings, starting from our proteomics studies on IPF, suggest that systemic involvement with altered molecular mechanisms and metabolic disorder is an underlying cause of fibrosis. The role of metabolic dysregulation in the pathogenesis of IPF has not been extensively studied, despite a recent surge of interest. In particular, our studies on bronchoalveolar lavage fluid have shown that the renin-angiotensin-aldosterone system (RAAS), the hypoxia/oxidative stress response, and changes in iron and lipid metabolism are involved in onset of IPF. These processes appear to interact in an intricate manner and to be related to different fibrosing pathologies not directly linked to the lung environment. The disordered metabolism of carbohydrates, lipids, proteins and hormones has been documented in lung, liver, and kidney fibrosis. Correcting these metabolic alterations may offer a new strategy for treating fibrosis. This paper focuses on the role of metabolic dysregulation in the pathogenesis of IPF and is a continuation of our previous studies, investigating metabolic dysregulation as a new target for fibrosis therapy.
Keywords: idiopathic pulmonary fibrosis; iron metabolism; lipid metabolism; metabolic dysregulation; oxidative stress; renin–angiotensin–aldosterone system.
Conflict of interest statement
All authors declare that they do not have any potential conflicts of interest.
Figures


References
-
- Joo S., Kim D.K., Sim H.J., Lee G.D., Hwang S.K., Choi S., Kim H.R., Kim Y.-H., Park S.-I. Clinical results of sublobar resection versus lobectomy or more extensive resection for lung cancer patients with idiopathic pulmonary fibrosis. J. Thorac. Dis. 2016;8:977–984. doi: 10.21037/jtd.2016.03.76. - DOI - PMC - PubMed
-
- Khalil W., Xia H., Bodempudi V., Kahm J., Hergert P., Smith K., Peterson M., Parker M., Herrera J., Bitterman P.B., et al. Pathologic Regulation of Collagen I by an Aberrant Protein Phosphatase 2A/Histone Deacetylase C4/MicroRNA-29 Signal Axis in Idiopathic Pulmonary Fibrosis Fibroblasts. Am. J. Respir. Cell Mol. Biol. 2015;53:391–399. doi: 10.1165/rcmb.2014-0150OC. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

