Characterization of Fe(III) Adsorption onto Zeolite and Bentonite
- PMID: 32784702
- PMCID: PMC7460527
- DOI: 10.3390/ijerph17165718
Characterization of Fe(III) Adsorption onto Zeolite and Bentonite
Abstract
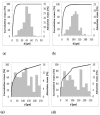
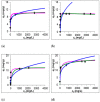
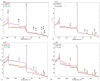
In this study, the adsorption of Fe(III) from aqueous solution on zeolite and bentonite was investigated by combining batch adsorption technique, Atomic adsorption spectroscopy, X-ray diffraction, and X-ray photoelectron spectroscopy analyses. Although iron is commonly found in water and is an essential bioelement, many industrial processes require efficient removal of iron from water. Two types of zeolite and two types of bentonite were used. The results showed that the maximum adsorption capacities for removal of Fe (III) by Zeolite Micro 20, Zeolite Micro 50, blue bentonite, and brown bentonite were 10.19, 9.73, 11.64, and 16.65 mg.g-1, respectively. Based on the X-ray photoelectron spectroscopy (XPS) and X-ray fluorescence (XRF) analyses of the raw samples and the solid residues after sorption at low and high initial Fe concentrations, the Fe content is different in the surface layer and in the bulk of the material. In the case of lower initial Fe concentration (200 mg.dm-3), more than 95% of Fe is adsorbed in the surface layer. In the case of higher initial Fe concentration (4000 mg.dm-3), only about 45% and 61% of Fe is adsorbent in the surface layer of zeolite and bentonite, respectively; the rest is adsorbed in deeper layers.
Keywords: Fe (III) removal; adsorption; bentonite; surface layer; zeolite.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Edwards M., McNeill L.S. Effect of phosphate inhibitors on lead release from pipes. J. AWWA. 2002;94:79. doi: 10.1002/j.1551-8833.2002.tb09383.x. - DOI
-
- Das S., Mishra S. Insight into the isotherm modelling, kinetic and thermodynamic exploration of iron adsorption from aqueous media by activated carbon developed from Limonia acidissima shell. Mater. Chem. Phys. 2020;245:122751. doi: 10.1016/j.matchemphys.2020.122751. - DOI
-
- Kang Y.-G., Vu H.C., Chang Y.-Y., Chang Y.-S. Fe(III) adsorption on graphene oxide: A low-cost and simple modificationmethod for persulfate activation. Chem. Eng. J. 2020;387:124012. doi: 10.1016/j.cej.2020.124012. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

