Separation of Radionuclides from Spent Decontamination Fluids via Adsorption onto Titanium Dioxide Nanotubes after Photocatalytic Degradation
- PMID: 32784733
- PMCID: PMC7466533
- DOI: 10.3390/nano10081553
Separation of Radionuclides from Spent Decontamination Fluids via Adsorption onto Titanium Dioxide Nanotubes after Photocatalytic Degradation
Abstract
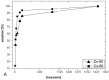
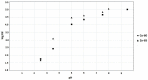
A one-step process combining the photocatalytic degradation of radionuclide complexes and the adsorption of liberated radionuclides on titanium dioxide nanotubes was developed and tested for the purification of aqueous waste produced from chemical decontamination of nuclear power plant circuit components. Among the tested forms of TiO2, only nanotubes exhibit both high photocatalytic activity and sorption ability, which support their application in a one-step purification process. The obtained results indicate that the photocatalytic degradation of complexes followed by the sorption of the radionuclides onto TiO2 nanotubes offers a promising route for treating spent decontamination fluids.
Keywords: decontamination fluids; photocatalytic degradation; titanium dioxide; titanium nanotubes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lieser K.H. Nuclear and Radiochemistry: Fundamentals and Applications. Wiley; Weinheim, Germany: 2001.
-
- International Atomic Energy Agency . Decontamination of Water Cooled Reactors. IAEA; Vienna, Austria: 1994. p. 81. Technical Report Ser. No. 365.
-
- Rosikova K., Sebesta F., John J., Buchtova K., Motl A. Separation of radionuclides from spent decontamination solutions onto selective inorganic-organic composite absorbers. Czechoslov. J. Phys. 2003;53:A603–A610. doi: 10.1007/s10582-003-0078-8. - DOI
-
- Bilewicz A., Dybczyński R., Narbutt J. Ion exchange of some transition metal cations on hydrated titanium dioxide in aqueous ammonia solutions. J. Radioanal. Nucl. Chem. 1992;15:273–282. doi: 10.1007/BF02047114. - DOI
-
- Danacikova E., John J., Motl A., Sebesta F., Hooper E.W. Study of sorption properties of various titanium dioxide materials. Czechoslov. J. Phys. 1999;49:789–795. doi: 10.1007/s10582-999-1064-6. - DOI
LinkOut - more resources
Full Text Sources

