Autophagy Declines with Premature Skin Aging resulting in Dynamic Alterations in Skin Pigmentation and Epidermal Differentiation
- PMID: 32784909
- PMCID: PMC7460956
- DOI: 10.3390/ijms21165708
Autophagy Declines with Premature Skin Aging resulting in Dynamic Alterations in Skin Pigmentation and Epidermal Differentiation
Abstract
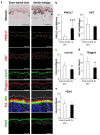
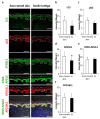
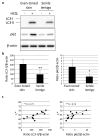
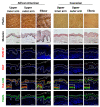
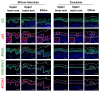
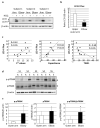
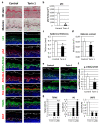
Autophagy is a membrane traffic system that provides sustainable degradation of cellular components for homeostasis, and is thus considered to promote health and longevity, though its activity declines with aging. The present findings show deterioration of autophagy in association with premature skin aging. Autophagy flux was successfully determined in skin tissues, which demonstrated significantly decreased autophagy in hyperpigmented skin such as that seen in senile lentigo. Furthermore, an exacerbated decline in autophagy was confirmed in xerotic hyperpigmentation areas, accompanied by severe dehydration and a barrier defect, which showed correlations with skin physiological conditions. The enhancement of autophagy in skin ex vivo ameliorated skin integrity, including pigmentation and epidermal differentiation. The present results indicate that the restoration of autophagy can contribute to improving premature skin aging by various intrinsic and extrinsic factors via the normalization of protein homeostasis.
Keywords: aging; autophagy; hyperpigmentation; keratinocyte; melanosome; skin.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

