New Horizons in Chemical Functionalization of Endohedral Metallofullerenes
- PMID: 32784953
- PMCID: PMC7463479
- DOI: 10.3390/molecules25163626
New Horizons in Chemical Functionalization of Endohedral Metallofullerenes
Abstract
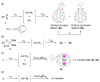
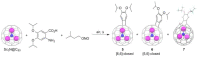
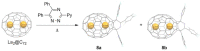
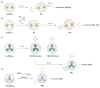
This overview explains some new aspects of chemical functionalization of endohedral metallofullerenes (EMFs) that have been unveiled in recent years. After differences in chemical reactivity between EMFs and the corresponding empty fullerenes are discussed, cage-opening reactions of EMFs are examined. Then, the selective bisfunctionalization of EMFs is explained. Finally, single-bonding derivatization of EMFs is addressed. The diversity and applicability of the chemical functionalization of endohedral metallofullerenes are presented to readers worldwide.
Keywords: addition reactions; carbenes; chemical functionalization; electrochemistry; endohedral metallofullerene; fullerene; lanthanide ions; nanocarbon; pericyclic reactions; radicals.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

References
-
- Heath J.R., O’Brien S.C., Zhang Q., Liu Y., Curl R.F., Kroto H.W., Tittel F.K., Smalley R.E. Lanthanum complexes of spheroidal carbon shells. J. Am. Chem. Soc. 1985;107:7779–7780. doi: 10.1021/ja00311a102. - DOI
-
- Shinohara H. Endohedral metallofullerenes. Rep. Prog. Phys. 2000;63:843–892. doi: 10.1088/0034-4885/63/6/201. - DOI
-
- Akasaka T., Nagase S. Endofullerenes: A New Family of Carbon Clusters. Kluwer; Dordrecht, The Netherlands: 2002.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

