Generation and Characterization of a DNA-GCN4 Oligonucleotide-Peptide Conjugate: The Impact DNA/Protein Interactions on the Sensitization of DNA
- PMID: 32784992
- PMCID: PMC7466028
- DOI: 10.3390/molecules25163630
Generation and Characterization of a DNA-GCN4 Oligonucleotide-Peptide Conjugate: The Impact DNA/Protein Interactions on the Sensitization of DNA
Abstract
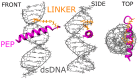
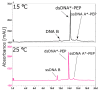
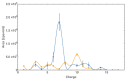
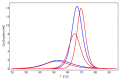
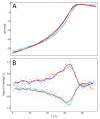
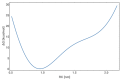
Radiotherapy, the most common therapy for the treatment of solid tumors, exerts its effects by inducing DNA damage. To fully understand the extent and nature of this damage, DNA models that mimic the in vivo situation should be utilized. In a cellular context, genomic DNA constantly interacts with proteins and these interactions could influence both the primary radical processes (triggered by ionizing radiation) and secondary reactions, ultimately leading to DNA damage. However, this is seldom addressed in the literature. In this work, we propose a general approach to tackle these shortcomings. We synthesized a protein-DNA complex that more closely represents DNA in the physiological environment than oligonucleotides solution itself, while being sufficiently simple to permit further chemical analyses. Using click chemistry, we obtained an oligonucleotide-peptide conjugate, which, if annealed with the complementary oligonucleotide strand, forms a complex that mimics the specific interactions between the GCN4 protein and DNA. The covalent bond connecting the oligonucleotide and peptide constitutes a part of substituted triazole, which forms due to the click reaction between the short peptide corresponding to the specific amino acid sequence of GCN4 protein (yeast transcription factor) and a DNA fragment that is recognized by the protein. DNAse footprinting demonstrated that the part of the DNA fragment that specifically interacts with the peptide in the complex is protected from DNAse activity. Moreover, the thermodynamic characteristics obtained using differential scanning calorimetry (DSC) are consistent with the interaction energies calculated at the level of metadynamics. Thus, we present an efficient approach to generate a well-defined DNA-peptide conjugate that mimics a real DNA-peptide complex. These complexes can be used to investigate DNA damage under conditions very similar to those present in the cell.
Keywords: DNA-protein interactions; radiotherapy; sensitizers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Bell S.P., Gann A., Levine M.R., Losick J.D., Watson T.A. Molecular Biology of the Gene. 7th ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY, USA: 2008. Baker. Chapter II. Structure and study of macromolecules.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

