Eight years of the East African Community Medicines Regulatory Harmonization initiative: Implementation, progress, and lessons learned
- PMID: 32785219
- PMCID: PMC7423058
- DOI: 10.1371/journal.pmed.1003134
Eight years of the East African Community Medicines Regulatory Harmonization initiative: Implementation, progress, and lessons learned
Abstract
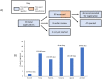
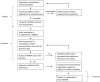
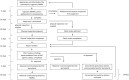
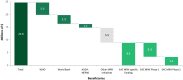
Jane H. Mashingia and colleagues reveal the progress made to date for the East African Community Medicines Regulatory Harmonization initiative.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: DH, DM, & ML declare they are employees of the Bill & Melinda Gates Foundation, which funds the EAC MRH initiative.
Figures
References
-
- Assessing national medicines regulatory systems. World Health Organization website. 2020 [2020 cited Feb 24]. https://www.who.int/medicines/areas/quality_safety/regulation_legislatio....
-
- Avastin approval history. 2019 [2019 cited Apr 22]. https://www.drugs.com/history/avastin.html
-
- Herceptin approval history. 2019 [cited 2019 Apr 22]. https://www.drugs.com/history/herceptin.html
-
- WHO Model List of Essential Medicines. 19th list. Geneva: World Health Organization, 2015.
-
- Kamwanja L, Saka J, Awotedu A, Fute I, Chamdimba C, Ndomondo-Sigonda M. Situational Analysis Study on Medicines Registration Harmonisation in Africa: Final Report for the East African Community. NEPAD, 2010.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

