Pretreatment and Acquired Antiretroviral Drug Resistance Among Persons Living With HIV in Four African Countries
- PMID: 32785695
- PMCID: PMC8492117
- DOI: 10.1093/cid/ciaa1161
Pretreatment and Acquired Antiretroviral Drug Resistance Among Persons Living With HIV in Four African Countries
Abstract
Background: Emerging HIV drug resistance (HIVDR) could jeopardize the success of standardized HIV management protocols in resource-limited settings. We characterized HIVDR among antiretroviral therapy (ART)-naive and experienced participants in the African Cohort Study (AFRICOS).
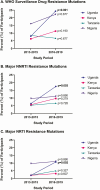
Methods: From January 2013 to April 2019, adults with HIV-1 RNA >1000 copies/mL underwent ART history review and HIVDR testing upon enrollment at 12 clinics in Uganda, Kenya, Tanzania, and Nigeria. We calculated resistance scores for specific drugs and tallied major mutations to non-nucleoside reverse transcriptase inhibitors (NNRTIs), nucleoside reverse transcriptase inhibitors (NRTIs), and protease inhibitors (PIs) using Stanford HIVDB 8.8 and SmartGene IDNS software. For ART-naive participants, World Health Organization surveillance drug resistance mutations (SDRMs) were noted.
Results: HIVDR testing was performed on 972 participants with median age 35.7 (interquartile range [IQR] 29.7-42.7) years and median CD4 295 (IQR 148-478) cells/mm3. Among 801 ART-naive participants, the prevalence of SDRMs was 11.0%, NNRTI mutations 8.2%, NRTI mutations 4.7%, and PI mutations 0.4%. Among 171 viremic ART-experienced participants, NNRTI mutation prevalence was 83.6%, NRTI 67.8%, and PI 1.8%. There were 90 ART-experienced participants with resistance to both efavirenz and lamivudine, 33 (36.7%) of whom were still prescribed these drugs. There were 10 with resistance to both tenofovir and lamivudine, 8 (80.0%) of whom were prescribed these drugs.
Conclusions: Participants on failing ART regimens had a high burden of HIVDR that potentially limited the efficacy of standardized first- and second-line regimens. Management strategies that emphasize adherence counseling while delaying ART switch may promote drug resistance and should be reconsidered.
Keywords: Africa South of the Sahara; acquired immunodeficiency syndrome; drug resistance; public health surveillance.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures


References
-
- Joint United Nations Programme on HIV/AIDS. Fact sheet—global AIDS update 2019. Available at: https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_.... Accessed 6 September 2019.
-
- Forsythe SS, McGreevey W, Whiteside A, et al. . Twenty years of antiretroviral therapy for people living with hiv: global costs, health achievements, economic benefits. Health Aff (Millwood) 2019; 38:1163–72. - PubMed
-
- Bennett DE, Myatt M, Bertagnolio S, Sutherland D, Gilks CF. Recommendations for surveillance of transmitted HIV drug resistance in countries scaling up antiretroviral treatment. Antivir Ther 2008; 13(Suppl 2):25–36. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

