Novel pathways of inflammation in human fetal membranes associated with preterm birth and preterm pre-labor rupture of the membranes
- PMID: 32785751
- PMCID: PMC9296260
- DOI: 10.1007/s00281-020-00808-x
Novel pathways of inflammation in human fetal membranes associated with preterm birth and preterm pre-labor rupture of the membranes
Abstract
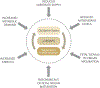
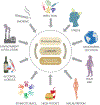
Spontaneous preterm birth (PTB) and preterm pre-labor rupture of the membranes (pPROM) are major pregnancy complications. Although PTB and pPROM have common etiologies, they arise from distinct pathophysiologic pathways. Inflammation is a common underlying mechanism in both conditions. Balanced inflammation is required for fetoplacental growth; however, overwhelming inflammation (physiologic at term and pathologic at preterm) can lead to term and preterm parturition. A lack of effective strategies to control inflammation and reduce the risk of PTB and pPROM suggests that there are several modes of the generation of inflammation which may be dependent on the type of uterine tissue. The avascular fetal membrane (amniochorion), which provides structure, support, and protection to the intrauterine cavity, is one of the key contributors of inflammation. Localized membrane inflammation helps tissue remodeling during pregnancy. Two unique mechanisms that generate balanced inflammation are the progressive development of senescence (aging) and cyclic cellular transitions: epithelial to mesenchymal (EMT) and mesenchymal to epithelial (MET). The intrauterine build-up of oxidative stress at term or in response to risk factors (preterm) can accelerate senescence and promote a terminal state of EMT, resulting in the accumulation of inflammation. Inflammation degrades the matrix and destabilizes membrane function. Inflammatory mediators from damaged membranes are propagated via extracellular vesicles (EV) to maternal uterine tissues and transition quiescent maternal uterine tissues into an active state of labor. Membrane inflammation and its propagation are fetal signals that may promote parturition. This review summarizes the mechanisms of fetal membrane cellular senescence, transitions, and the generation of inflammation that contributes to term and preterm parturitions.
Keywords: Amniochorion; Cytokines; EMT; Exosome; Fetal membranes; Inflammation; Mesenchymal cells; Progesterone; p38MAPK.
Conflict of interest statement
Figures


References
-
- Adams Waldorf KM, Singh N, Mohan AR, Young RC, Ngo L, Das A, … Johnson MR (2015). Uterine overdistention induces preterm labor mediated by inflammation: observations in pregnant women and nonhuman primates. Am J Obstet Gynecol, 213(6), 830.e831–830.e819. doi: 10.1016/j.ajog.2015.08.028 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

