Evidence of potent humoral immune activity in COVID-19-infected kidney transplant recipients
- PMID: 32786152
- PMCID: PMC7436882
- DOI: 10.1111/ajt.16261
Evidence of potent humoral immune activity in COVID-19-infected kidney transplant recipients
Abstract
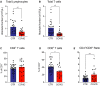
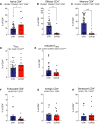
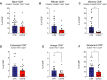
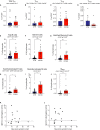
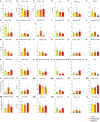
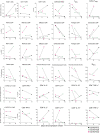
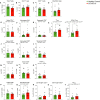
Whether kidney transplant recipients are capable of mounting an effective anti-severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) adaptive immune response despite chronic immunosuppression is unknown and has important implications for therapy. Herein, we analyzed peripheral blood cell surface and intracellular cytokine phenotyping by flow cytometry along with serum antibody testing in 18 kidney transplant recipients with active coronavirus disease 2019 (COVID-19) infection and 36 matched, transplanted controls without COVID-19. We observed significantly fewer total lymphocytes and fewer circulating memory CD4+ and CD8+ T cells in the COVID-19 subjects. We also showed fewer anergic and senescent CD8+ T cells in COVID-19 individuals, but no differences in exhausted CD8+ T cells, nor in any of these CD4+ T cell subsets between groups. We also observed greater frequencies of activated B cells in the COVID-19 patients. Sixteen of 18 COVID-19 subjects tested for anti-SARS-CoV-2 serum antibodies showed positive immunoglobulin M or immunoglobulin G titers. Additional analyses showed no significant correlation among immune phenotypes and degrees of COVID-19 disease severity. Our findings indicate that immunosuppressed kidney transplant recipients admitted to the hospital with acute COVID-19 infection can mount SARS-CoV-2-reactive adaptive immune responses. The findings raise the possibility that empiric reductions in immunosuppressive therapy for all kidney transplant recipients with active COVID-19 may not be required.
Keywords: immune regulation; immunobiology; immunosuppressant - other; immunosuppression/immune modulation; infection and infectious agents - viral; kidney transplantation/nephrology; translational research/science.
© 2020 The American Society of Transplantation and the American Society of Transplant Surgeons.
Figures
Comment in
-
Three platforms: Ways to pivot in a pandemic.Am J Transplant. 2021 Mar;21(3):911-912. doi: 10.1111/ajt.16514. Am J Transplant. 2021. PMID: 33641267 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

