Investigating the Effects of Lithium Phosphorous Oxynitride Coating on Blended Solid Polymer Electrolytes
- PMID: 32786244
- PMCID: PMC10905425
- DOI: 10.1021/acsami.0c09113
Investigating the Effects of Lithium Phosphorous Oxynitride Coating on Blended Solid Polymer Electrolytes
Abstract
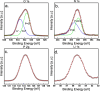
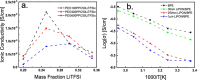
Solid-state electrolytes are very promising to enhance the safety of lithium-ion batteries. Two classes of solid electrolytes, polymer and ceramic, can be combined to yield a hybrid electrolyte that can synergistically combine the properties of both materials. Chemical stability, thermal stability, and high mechanical modulus of ceramic electrolytes against dendrite penetration can be combined with the flexibility and ease of processing of polymer electrolytes. By coating a polymer electrolyte with a ceramic electrolyte, the stability of the solid electrolyte is expected to improve against lithium metal, and the ionic conductivity could remain close to the value of the original polymer electrolyte, as long as an appropriate thickness of the ceramic electrolyte is applied. Here, we report a bilayered lithium-ion conducting hybrid solid electrolyte consisting of a blended polymer electrolyte (BPE) coated with a thin layer of the inorganic solid electrolyte lithium phosphorous oxynitride (LiPON). The hybrid system was thoroughly studied. First, we investigated the influence of the polymer chain length and lithium salt ratio on the ionic conductivity of the BPE based on poly(ethylene oxide) (PEO) and poly(propylene carbonate) (PPC) with the salt lithium bis(trifluoromethanesulfonyl)imide (LiTFSI). The optimized BPE consisted of 100 k molecular weight PEO, 50 k molecular weight PPC, and 25(w/w)% LiTFSI, (denoted as PEO100PPC50LiTFSI25), which exhibited an ionic conductivity of 2.11 × 10-5 S/cm, and the ionic conductivity showed no thermal memory effects as the PEO crystallites were well disrupted by PPC and LiTFSI. Second, the effects of LiPON coating on the BPE were evaluated as a function of thickness down to 20 nm. The resulting bilayer structure showed an increase in the voltage window from 5.2 to 5.5 V (vs Li/Li+) and thermal activation energies that approached the activation energy of the BPE when thinner LiPON layers were used, resulting in similar ionic conductivities for 30 nm LiPON coatings on PEO100PPC50LiTFSI25. Coating BPEs with a thin layer of LiPON is shown to be an effective strategy to improve the long-term stability against lithium.
Keywords: LiPON; critical thickness; hybrid bilayer electrolyte; lithium-ion batteries; molecular weight; solid-state.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








References
-
- Chen R.; Qu W.; Guo X.; Li L.; Wu F. The Pursuit of Solid-State Electrolytes for Lithium Batteries: From Comprehensive Insight to Emerging Horizons. Mater. Horiz. 2016, 3, 487–516.
-
- Zhang J.; Zang X.; Wen H.; Dong T.; Chai J.; Li Y.; Chen B.; Zhao J.; Dong S.; Ma J.; Yue L. P.; Liu Z. H.; Guo X. X.; Cui G. L.; Chen L. Q. High-Voltage and Free-Standing Poly(Propylene Carbonate)/Li6.75La3Zr1.75Ta0.25O12 Composite Solid Electrolyte for Wide Temperature Range and Flexible Solid Lithium Ion Battery. J. Mater. Chem. A 2017, 5, 4940–4948.
-
- Kim J. G.; Son B.; Mukherjee S.; Schuppert N.; Bates A.; Kwon O.; Choi M. J.; Chung H. Y.; Park S. A Review of Lithium and Non-Lithium Based Solid State Batteries. J. Power Sources 2015, 282, 299–322.
LinkOut - more resources
Full Text Sources
Miscellaneous

