A Chemoenzymatic Synthesis of the (RP)-Isomer of the Antiviral Prodrug Remdesivir
- PMID: 32786401
- PMCID: PMC7418565
- DOI: 10.1021/acs.biochem.0c00591
A Chemoenzymatic Synthesis of the (RP)-Isomer of the Antiviral Prodrug Remdesivir
Abstract
The COVID-19 pandemic threatens to overwhelm healthcare systems around the world. The only current FDA-approved treatment, which directly targets the virus, is the ProTide prodrug remdesivir. In its activated form, remdesivir prevents viral replication by inhibiting the essential RNA-dependent RNA polymerase. Like other ProTide prodrugs, remdesivir contains a chiral phosphorus center. The initial selection of the (SP)-diastereomer for remdesivir was reportedly due to the difficulty in producing the pure (RP)-diastereomer of the required precursor. However, the two currently known enzymes responsible for the initial activation step of remdesivir are each stereoselective and show differential tissue distribution. Given the ability of the COVID-19 virus to infect a wide array of tissue types, inclusion of the (RP)-diastereomer may be of clinical significance. To help overcome the challenge of obtaining the pure (RP)-diastereomer of remdesivir, we have developed a novel chemoenzymatic strategy that utilizes a stereoselective variant of the phosphotriesterase from Pseudomonas diminuta to enable the facile isolation of the pure (RP)-diastereomer of the chiral precursor for the chemical synthesis of the (RP)-diastereomer of remdesivir.
Conflict of interest statement
Notes
Authors declare no competing financial interest.
Figures
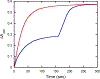
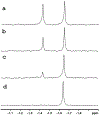
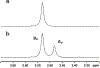
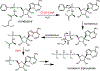
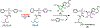
References
-
- Johns Hopkins University (2020) CORONAVIRUS RESOURCE CENTER, https://coronavirus.jhu.edu/, Accessed: 7-1-2020.
-
- Wang Q, Wu J, Wang H, Gao Y, Liu Q, Mu A, Ji W, Yan L, Zhu Y, Zhu C, Fang X, Yang X, Huang Y, Gao H, Liu F, Ge J, Sun Q, Yang X, Xu W, Liu Z, Yang H, Lou Z, Jiang B, Guddat LW, Gong P, and Rao Z (2020) Structural Basis for RNA Replication by the SARS-CoV-2 Polymerase, Cell. 182, 417–428. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

