Synthesis of Phenacene-Helicene Hybrids by Directed Remote Metalation
- PMID: 32786610
- PMCID: PMC7498163
- DOI: 10.1021/acs.joc.0c01097
Synthesis of Phenacene-Helicene Hybrids by Directed Remote Metalation
Abstract
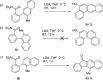
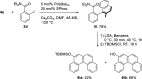
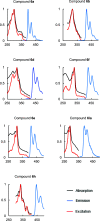
Polycyclic aromatic hydrocarbons (PAHs) with six and seven rings were synthesized via directed metalation and cross-coupling of chrysenyl N,N-diethyl carboxamides with o-tolyl and methylnaphthalenyl derivatives. In the presence of competing ortho sites, the site selectivity in iodination of chrysenyl amides by directed ortho metalation (DoM) was influenced by the lithium base. The catalyst ligand bite angle was presumably important in the cross-coupling of sterically hindered bulky PAHs. Subsequent directed remote metalation of biaryls under standard conditions and at elevated temperatures afforded various fused six- and seven-ring PAHs, all in good yields and with fluorescent properties.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
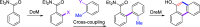


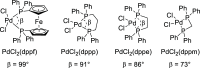

References
-
- Karras M.; Dąbrowski M.; Pohl R.; Rybáček J.; Vacek J.; Bednárová L.; Grela K.; Starý I.; Stará I. G.; Schmidt B. Helicenes as Chirality-Inducing Groups in Transition-Metal Catalysis: The First Helically Chiral Olefin Metathesis Catalyst. Chem.—Eur. J. 2018, 24, 10994–10998. 10.1002/chem.201802786. - DOI - PubMed
-
- Takaishi K.; Iwachido K.; Takehana R.; Uchiyama M.; Ema T. Evolving Fluorophores into Circularly Polarized Luminophores with a Chiral Naphthalene Tetramer: Proposal of Excimer Chirality Rule for Circularly Polarized Luminescence. J. Am. Chem. Soc. 2019, 141, 6185–6190. 10.1021/jacs.9b02582. - DOI - PubMed
LinkOut - more resources
Full Text Sources

