Computational Analysis of Enantioselective Pd-Catalyzed α-Arylation of Ketones
- PMID: 32786644
- PMCID: PMC8009508
- DOI: 10.1021/acs.joc.0c01768
Computational Analysis of Enantioselective Pd-Catalyzed α-Arylation of Ketones
Abstract
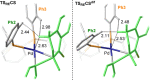
The direct α-arylation of carbonyl compounds emerged over the last two decades as a straightforward method for the formation of C(sp3)-C(sp2) bonds. Mechanistic studies suggested a classical cross-coupling catalytic cycle. This consists of oxidative addition of the aryl halide (ArX) to the Pd(0)-catalyst, transmetallation of the Na- or K-enolate generated in situ, and subsequent reductive elimination. Even though the general reaction mechanism was thoroughly investigated, studies focusing on enantioselective variants of this transformation are rare. Here, the computational study of the [Pd(BINAP)]-catalyzed α-arylation of 2-methyltetralone with bromobenzene is reported. The whole reaction energy profile was computed and several mechanistic scenarios were investigated for the key steps of the reaction, which are the enolate transmetallation and the C-C bond-forming reductive elimination. Among the computed mechanisms, the reductive elimination from the C-bound enolate Pd complex was found to be the most favorable one, providing a good match with the stereoselectivity observed experimentally with different ligands and substrates. Detailed analysis of the stereodetermining transition structures allowed us to establish the origin of the reaction enantioselectivity.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Palucki M.; Buchwald S. L. Palladium-Catalyzed α-Arylation of Ketones. J. Am. Chem. Soc. 1997, 119, 11108.10.1021/ja972593s. - DOI
-
- Hamann B. C.; Hartwig J. F. Palladium-Catalyzed Direct α-Arylation of Ketones. Rate Acceleration by Sterically Hindered Chelating Ligands and Reductive Elimination from a Transition Metal Enolate Complex. J. Am. Chem. Soc. 1997, 119, 12382.10.1021/ja9727880. - DOI
Publication types
LinkOut - more resources
Full Text Sources

