Nanoscale Spatial Distribution of Supported Nanoparticles Controls Activity and Stability in Powder Catalysts for CO Oxidation and Photocatalytic H2 Evolution
- PMID: 32786792
- PMCID: PMC7924732
- DOI: 10.1021/jacs.0c03842
Nanoscale Spatial Distribution of Supported Nanoparticles Controls Activity and Stability in Powder Catalysts for CO Oxidation and Photocatalytic H2 Evolution
Abstract
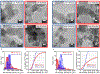
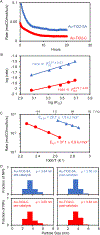
Supported metal nanoparticles are essential components of high-performing catalysts, and their structures are intensely researched. In comparison, nanoparticle spatial distribution in powder catalysts is conventionally not quantified, and the influence of this collective property on catalyst performance remains poorly investigated. Here, we demonstrate a general colloidal self-assembly method to control uniformity of nanoparticle spatial distribution on common industrial powder supports. We quantify distributions on the nanoscale using image statistics and show that the type of nanospatial distribution determines not only the stability, but also the activity of heterogeneous catalysts. Widely investigated systems (Au-TiO2 for CO oxidation thermocatalysis and Pd-TiO2 for H2 evolution photocatalysis) were used to showcase the universal importance of nanoparticle spatial organization. Spatially and temporally resolved microkinetic modeling revealed that nonuniformly distributed Au nanoparticles suffer from local depletion of surface oxygen, and therefore lower CO oxidation activity, as compared to uniformly distributed nanoparticles. Nanoparticle spatial distribution also determines the stability of Pd-TiO2 photocatalysts, because nonuniformly distributed nanoparticles sinter while uniformly distributed nanoparticles do not. This work introduces new tools to evaluate and understand catalyst collective (ensemble) properties in powder catalysts, which thereby pave the way to more active and stable heterogeneous catalysts.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
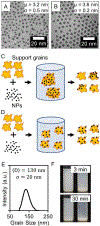


References
-
- Hvolbaek B; Janssens TVW; Clausen BS; Falsig H; Christensen CH; Norskov JK Catalytic Activity of Au Nanoparticles. Nano Today 2007, 2 (4), 14–18.
-
- Corma A; Garcia H Supported Gold Nanoparticles as Catalysts for Organic Reactions. Chem. Soc. Rev. 2008, 37 (9), 2096–2126. - PubMed
-
- Munnik P; de Jongh PE; de Jong KP Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115 (14), 6687–6718. - PubMed
-
- Losch P; Huang W; Goodman ED; Wrasman CJ; Holm A; Riscoe AR; Schwalbe JA; Cargnello M Colloidal Nanocrystals for Heterogeneous Catalysis. Nano Today 2019, 24, 15–47.
-
- Nie Z; Petukhova A; Kumacheva E Properties and Emerging Applications of Self-Assembled Structures Made from Inorganic Nanoparticles. Nat. Nanotechnol. 2010, 5 (1), 15–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

