Development and evaluation of an up-converting phosphor technology-based lateral flow assay for rapid and quantitative detection of Coxiella burnetii phase I strains
- PMID: 32787788
- PMCID: PMC7425161
- DOI: 10.1186/s12866-020-01934-0
Development and evaluation of an up-converting phosphor technology-based lateral flow assay for rapid and quantitative detection of Coxiella burnetii phase I strains
Abstract
Background: Coxiella burnetii is an obligate intracellular Gram-negative bacterium that causes a zoonotic disease commonly called Q fever globally. In this study, an up-converting phosphor technology-based lateral flow (UPT-LF) assay was established for the rapid and specific detection of phase I strains of C. burnetii.
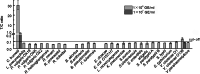
Results: Specific monoclonal antibodies (10B5 and 10G7) against C. burnetii phase I strains were prepared and selected for use in the UPT-LF assay by the double-antibody-sandwich method. The detection sensitivity of the Coxiella-UPT-LF was 5 × 104 GE/ml for a purified C. burnetii phase I strain and 10 ng/ml for LPS of C. burnetii Nine Mile phase I (NMI). Good linearity was observed for C. burnetii phase I and NMI LPS quantification (R2 ≥ 0.989). The UPT-LF assay also exhibited a high specificity to C. burnetii, without false-positive results even at 108 GE/ml of non-specific bacteria, and good inclusivity for detecting different phase I strains of C. burnetii. Moreover, the performance of the Coxiella-UPT-LF assay was further confirmed using experimentally and naturally infected samples.
Conclusions: Our results indicate that Coxiella-UPT-LF is a sensitive and reliable method for rapid screening of C. burnetii, suitable for on-site detection in the field.
Keywords: Coxiella burnetii; Lipopolysaccharide; Monoclonal antibody; Q fever; Up-converting phosphor technology-based lateral flow.
Conflict of interest statement
Author Dongsheng Zhou is an Associate Editor of this journal. The authors have no conflict of interest to declare.
Figures


Similar articles
-
Development of an up-converting phosphor technology-based 10-channel lateral flow assay for profiling antibodies against Yersinia pestis.J Microbiol Methods. 2010 Nov;83(2):133-40. doi: 10.1016/j.mimet.2010.08.005. Epub 2010 Aug 27. J Microbiol Methods. 2010. PMID: 20801166
-
Candidate antigens for Q fever serodiagnosis revealed by immunoscreening of a Coxiella burnetii protein microarray.Clin Vaccine Immunol. 2008 Dec;15(12):1771-9. doi: 10.1128/CVI.00300-08. Epub 2008 Oct 8. Clin Vaccine Immunol. 2008. PMID: 18845831 Free PMC article.
-
Humoral immune response to Q fever: enzyme-linked immunosorbent assay antibody response to Coxiella burnetii in experimentally infected guinea pigs.J Clin Microbiol. 1986 Dec;24(6):935-9. doi: 10.1128/jcm.24.6.935-939.1986. J Clin Microbiol. 1986. PMID: 3537005 Free PMC article.
-
Q fever: persistence of antigenic non-viable cell residues of Coxiella burnetii in the host--implications for post Q fever infection fatigue syndrome and other chronic sequelae.QJM. 2009 Oct;102(10):673-84. doi: 10.1093/qjmed/hcp077. Epub 2009 Jun 25. QJM. 2009. PMID: 19556396 Review.
-
Coxiella burnetii Lipopolysaccharide: What Do We Know?Int J Mol Sci. 2017 Nov 23;18(12):2509. doi: 10.3390/ijms18122509. Int J Mol Sci. 2017. PMID: 29168790 Free PMC article. Review.
Cited by
-
An up-converting phosphor technology-based lateral flow assay for rapid detection of major mycotoxins in feed: Comparison with enzyme-linked immunosorbent assay and high-performance liquid chromatography-tandem mass spectrometry.PLoS One. 2021 Apr 16;16(4):e0250250. doi: 10.1371/journal.pone.0250250. eCollection 2021. PLoS One. 2021. PMID: 33861782 Free PMC article.
-
A protein-protein interaction map reveals that the Coxiella burnetii effector CirB inhibits host proteasome activity.PLoS Pathog. 2022 Jul 11;18(7):e1010660. doi: 10.1371/journal.ppat.1010660. eCollection 2022 Jul. PLoS Pathog. 2022. PMID: 35816513 Free PMC article.
-
Development and multi-center clinical trials of an up-converting phosphor technology-based point-of-care (UPT-POCT) assay for rapid COVID-19 diagnosis and prediction of protective effects.BMC Microbiol. 2022 Feb 3;22(1):42. doi: 10.1186/s12866-022-02450-z. BMC Microbiol. 2022. PMID: 35114938 Free PMC article. Clinical Trial.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

