Targeting immune checkpoints in hematological malignancies
- PMID: 32787882
- PMCID: PMC7425174
- DOI: 10.1186/s13045-020-00947-6
Targeting immune checkpoints in hematological malignancies
Abstract
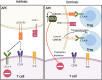
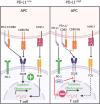
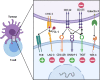
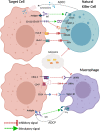
Immune checkpoint blockade (ICB) therapies such as anti-programmed death 1 (PD-1) and anti-CTLA-4 (cytotoxic T lymphocyte-associated protein 4) have dramatically transformed treatment in solid tumor oncology. While immunotherapeutic approaches such as stem cell transplantation and anti-cancer monoclonal antibodies have made critical contributions to improve outcomes in hematological malignancies, clinical benefits of ICB are observed in only limited tumor types that are particularly characterized by a high infiltration of immune cells. Importantly, even patients that initially respond to ICB are unable to achieve long-term disease control using these therapies. Indeed, primary and acquired resistance mechanisms are differentially orchestrated in hematological malignancies depending on tumor types and/or genotypes, and thus, an in-depth understanding of the disease-specific immune microenvironments will be essential in improving efficacy. In addition to PD-1 and CTLA-4, various T cell immune checkpoint molecules have been characterized that regulate T cell responses in a non-redundant manner. Several lines of evidence suggest that these T cell checkpoint molecules might play unique roles in hematological malignancies, highlighting their potential as therapeutic targets. Targeting innate checkpoint molecules on natural killer cells and/or macrophages has also emerged as a rational approach against tumors that are resistant to T cell-mediated immunity. Given that various monoclonal antibodies against tumor surface proteins have been clinically approved in hematological malignancies, innate checkpoint blockade might play a key role to augment antibody-mediated cellular cytotoxicity and phagocytosis. In this review, we discuss recent advances and emerging roles of immune checkpoint blockade in hematological malignancies.
Keywords: Hematological malignancy; Immune checkpoint molecule; Immunotherapy; Tumor microenvironment.
Conflict of interest statement
Mark J. Smyth has research agreements with Bristol Myers Squibb and Tizona Therapeutics and serves on the Scientific Advisory Board of Tizona Therapeutics and Compass Therapeutics.
Figures
References
-
- Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(11):1506–1517. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

