HIV-cell membrane fusion intermediates are restricted by Serincs as revealed by cryo-electron and TIRF microscopy
- PMID: 32788212
- PMCID: PMC7650252
- DOI: 10.1074/jbc.RA120.014466
HIV-cell membrane fusion intermediates are restricted by Serincs as revealed by cryo-electron and TIRF microscopy
Abstract
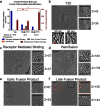
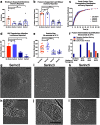
To enter a cell and establish infection, HIV must first fuse its lipid envelope with the host cell plasma membrane. Whereas the process of HIV membrane fusion can be tracked by fluorescence microscopy, the 3D configuration of proteins and lipids at intermediate steps can only be resolved with cryo-electron tomography (cryoET). However, cryoET of whole cells is technically difficult. To overcome this problem, we have adapted giant plasma membrane vesicles (or blebs) from native cell membranes expressing appropriate receptors as targets for fusion with HIV envelope glycoprotein-expressing pseudovirus particles with and without Serinc host restriction factors. The fusion behavior of these particles was probed by TIRF microscopy on bleb-derived supported membranes. Timed snapshots of fusion of the same particles with blebs were examined by cryo-ET. The combination of these methods allowed us to characterize the structures of various intermediates on the fusion pathway and showed that when Serinc3 or Serinc5 (but not Serinc2) were present, later fusion products were more prevalent, suggesting that Serinc3/5 act at multiple steps to prevent progression to full fusion. In addition, the antifungal amphotericin B reversed Serinc restriction, presumably by intercalation into the fusing membranes. Our results provide a highly detailed view of Serinc restriction of HIV-cell membrane fusion and thus extend current structural and functional information on Serinc as a lipid-binding protein.
Keywords: HIV; cryo-electron microscopy; host-pathogen interaction; human immunodeficiency virus (HIV); membrane fusion; single-particle tracking; virus entry.
© 2020 Ward et al.
Conflict of interest statement
Conflict of interest—The authors declare that they have no conflicts of interest with the contents of this article.
Figures


Comment in
-
HIV fusion: Catch me if you can.J Biol Chem. 2020 Nov 6;295(45):15196-15197. doi: 10.1074/jbc.H120.016022. J Biol Chem. 2020. PMID: 33158917 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

