Mitofusin 2 regulates neutrophil adhesive migration and the actin cytoskeleton
- PMID: 32788232
- PMCID: PMC7491649
- DOI: 10.1242/jcs.248880
Mitofusin 2 regulates neutrophil adhesive migration and the actin cytoskeleton
Abstract
Neutrophils rely on glycolysis for energy production. How mitochondria regulate neutrophil function is not fully understood. Here, we report that mitochondrial outer membrane protein Mitofusin 2 (MFN2) regulates neutrophil homeostasis and chemotaxis in vivoMfn2-deficient neutrophils are released from the hematopoietic tissue, trapped in the vasculature in zebrafish embryos, and not capable of chemotaxis. Consistent with this, human neutrophil-like cells that are deficient for MFN2 fail to arrest on activated endothelium under sheer stress or perform chemotaxis on 2D surfaces. Deletion of MFN2 results in a significant reduction of neutrophil infiltration to the inflamed peritoneal cavity in mice. Mechanistically, MFN2-deficient neutrophil-like cells display disrupted mitochondria-ER interaction, heightened intracellular Ca2+ levels and elevated Rac activation after chemokine stimulation. Restoring a mitochondria-ER tether rescues the abnormal Ca2+ levels, Rac hyperactivation and chemotaxis defect resulting from MFN2 depletion. Finally, inhibition of Rac activation restores chemotaxis in MFN2-deficient neutrophils. Taken together, we have identified that MFN2 regulates neutrophil migration via maintaining the mitochondria-ER interaction to suppress Rac activation, and uncovered a previously unrecognized role of MFN2 in regulating cell migration and the actin cytoskeleton.This article has an associated First Person interview with the first authors of the paper.
Keywords: Actin; Chemotaxis; Leukocyte; Mitochondria; Rac; Zebrafish.
© 2020. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures


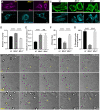
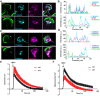


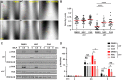
References
-
- Amini P., Stojkov D., Felser A., Jackson C. B., Courage C., Schaller A., Gelman L., Soriano M. E., Nuoffer J.-M., Scorrano L. et al. (2018). Neutrophil extracellular trap formation requires OPA1-dependent glycolytic ATP production. Nat. Commun. 9, 2958 10.1038/s41467-018-05387-y - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

