Neural transcription factor Pou4f1 promotes renal fibrosis via macrophage-myofibroblast transition
- PMID: 32788346
- PMCID: PMC7456094
- DOI: 10.1073/pnas.1917663117
Neural transcription factor Pou4f1 promotes renal fibrosis via macrophage-myofibroblast transition
Erratum in
-
Correction for Tang et al., Neural transcription factor Pou4f1 promotes renal fibrosis via macrophage-myofibroblast transition.Proc Natl Acad Sci U S A. 2022 Mar 8;119(10):e2200781119. doi: 10.1073/pnas.2200781119. Epub 2022 Mar 4. Proc Natl Acad Sci U S A. 2022. PMID: 35245173 Free PMC article. No abstract available.
Abstract
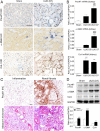
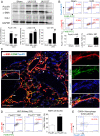
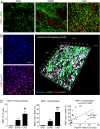
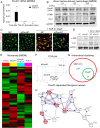
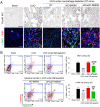
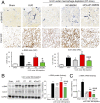
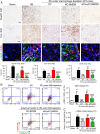
Unresolved inflammation can lead to tissue fibrosis and impaired organ function. Macrophage-myofibroblast transition (MMT) is one newly identified mechanism by which ongoing chronic inflammation causes progressive fibrosis in different forms of kidney disease. However, the mechanisms underlying MMT are still largely unknown. Here, we discovered a brain-specific homeobox/POU domain protein Pou4f1 (Brn3a) as a specific regulator of MMT. Interestingly, we found that Pou4f1 is highly expressed by macrophages undergoing MMT in sites of fibrosis in human and experimental kidney disease, identified by coexpression of the myofibroblast marker, α-SMA. Unexpectedly, Pou4f1 expression peaked in the early stage in renal fibrogenesis in vivo and during MMT of bone marrow-derived macrophages (BMDMs) in vitro. Mechanistically, chromatin immunoprecipitation (ChIP) assay identified that Pou4f1 is a Smad3 target and the key downstream regulator of MMT, while microarray analysis defined a Pou4f1-dependent fibrogenic gene network for promoting TGF-β1/Smad3-driven MMT in BMDMs at the transcriptional level. More importantly, using two mouse models of progressive renal interstitial fibrosis featuring the MMT process, we demonstrated that adoptive transfer of TGF-β1-stimulated BMDMs restored both MMT and renal fibrosis in macrophage-depleted mice, which was prevented by silencing Pou4f1 in transferred BMDMs. These findings establish a role for Pou4f1 in MMT and renal fibrosis and suggest that Pou4f1 may be a therapeutic target for chronic kidney disease with progressive renal fibrosis.
Keywords: Pou4f1; macrophage–myofibroblast transition; renal fibrosis.
Conflict of interest statement
The authors declare no competing interest.
Figures

Comment in
-
Uro-Science.J Urol. 2021 May;205(5):1517-1519. doi: 10.1097/JU.0000000000001658. Epub 2021 Feb 24. J Urol. 2021. PMID: 33625915 No abstract available.
References
-
- Rockey D. C., Bell P. D., Hill J. A., Fibrosis–A common pathway to organ injury and failure. N. Engl. J. Med. 372, 1138–1149 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

