Global variability of the human IgG glycome
- PMID: 32788422
- PMCID: PMC7467356
- DOI: 10.18632/aging.103884
Global variability of the human IgG glycome
Abstract
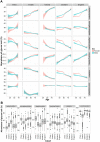
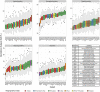
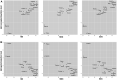
Immunoglobulin G (IgG) is the most abundant serum antibody which structural characteristics and effector functions are modulated through the attachment of various sugar moieties called glycans. Composition of the IgG N-glycome changes with age of an individual and in different diseases. Variability of IgG glycosylation within a population is well studied and is known to be affected by both genetic and environmental factors. However, global inter-population differences in IgG glycosylation have never been properly addressed. Here we present population-specific N-glycosylation patterns of IgG, analyzed in 5 different populations totaling 10,482 IgG glycomes, and of IgG's fragment crystallizable region (Fc), analyzed in 2,579 samples from 27 populations sampled across the world. Country of residence associated with many N-glycan features and the strongest association was with monogalactosylation where it explained 38% of variability. IgG monogalactosylation strongly correlated with the development level of a country, defined by United Nations health and socioeconomic development indicators, and with the expected lifespan. Subjects from developing countries had low levels of IgG galactosylation, characteristic for inflammation and ageing. Our results suggest that citizens of developing countries may be exposed to environmental factors that can cause low-grade chronic inflammation and the apparent increase in biological age.
Keywords: Fc glycosylation; aging; glycans; immunoglobulin G; mass spectrometry.
Conflict of interest statement
Figures
References
-
- Rispens T, Vidarsson G. Chapter 9 - Human IgG Subclasses. In: Antibody Fc. Linking Adaptive and Innate Immunity.. 2013; pp. 159–77. 10.1016/B978-0-12-394802-1.00009-1 - DOI
-
- Schur PH. IgG subclasses. A historical perspective. Monogr Allergy. 1988; 23:1–11. - PubMed
-
- Varki A, Gagneux P. Biological Functions of Glycans. 2017. In: Varki A, Cummings RD, Esko JD, Stanley P, Hart GW, Aebi M, Darvill AG, Kinoshita T, Packer NH, Prestegard JH, Schnaar RL, Seeberger PH, editors. Essentials of Glycobiology. 3rd ed. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2015–2017. Chapter 7. 10.1101/glycobiology.3e.007 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

