The JAK1/STAT3/SOCS3 axis in bone development, physiology, and pathology
- PMID: 32788655
- PMCID: PMC8080635
- DOI: 10.1038/s12276-020-0445-6
The JAK1/STAT3/SOCS3 axis in bone development, physiology, and pathology
Abstract
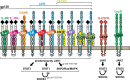
Bone growth and the maintenance of bone structure are controlled by multiple endocrine and paracrine factors, including cytokines expressed locally within the bone microenvironment and those that are elevated, both locally and systemically, under inflammatory conditions. This review focuses on those bone-active cytokines that initiate JAK-STAT signaling, and outlines the discoveries made from studying skeletal defects caused by induced or spontaneous modifications in this pathway. Specifically, this review describes defects in JAK1, STAT3, and SOCS3 signaling in mouse models and in humans, including mutations designed to modify these pathways downstream of the gp130 coreceptor. It is shown that osteoclast formation is generally stimulated indirectly by these pathways through JAK1 and STAT3 actions in inflammatory and other accessory cells, including osteoblasts. In addition, in bone remodeling, osteoblast differentiation is increased secondary to stimulated osteoclast formation through an IL-6-dependent pathway. In growth plate chondrocytes, STAT3 signaling promotes the normal differentiation process that leads to bone lengthening. Within the osteoblast lineage, STAT3 signaling promotes bone formation in normal physiology and in response to mechanical loading through direct signaling in osteocytes. This activity, particularly that of the IL-6/gp130 family of cytokines, must be suppressed by SOCS3 for the normal formation of cortical bone.
Conflict of interest statement
The author declares no conflict of interest.
Figures

References
-
- Shin HI, et al. Gp130-mediated signaling is necessary for normal osteoblastic function in vivo and in vitro. Endocrinology. 2004;145:1376–1385. - PubMed
-
- Kawasaki K, et al. Osteoclasts are present in gp130-deficient mice. Endocrinology. 1997;138:4959–4965. - PubMed
-
- Sims NA. Cardiotrophin-like cytokine factor 1 (CLCF1) and neuropoietin (NP) signalling and their roles in development, adulthood, cancer and degenerative disorders. Cytokine Growth Factor Rev. 2015;26:517–522. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

