Heat detection by the TRPM2 ion channel
- PMID: 32788732
- PMCID: PMC7613795
- DOI: 10.1038/s41586-020-2510-7
Heat detection by the TRPM2 ion channel
Figures
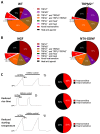
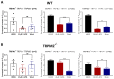
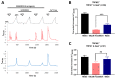
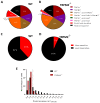
Comment in
-
Reply to: Heat detection by the TRPM2 ion channel.Nature. 2020 Aug;584(7820):E13-E15. doi: 10.1038/s41586-020-2511-6. Nature. 2020. PMID: 32788731 No abstract available.
Comment on
-
A TRP channel trio mediates acute noxious heat sensing.Nature. 2018 Mar 29;555(7698):662-666. doi: 10.1038/nature26137. Epub 2018 Mar 14. Nature. 2018. PMID: 29539642
References
-
- Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

