Efficacy and safety of second-generation CAR T-cell therapy in diffuse large B-cell lymphoma: A meta-analysis
- PMID: 32789017
- PMCID: PMC7416618
- DOI: 10.3892/mco.2020.2103
Efficacy and safety of second-generation CAR T-cell therapy in diffuse large B-cell lymphoma: A meta-analysis
Abstract
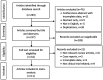
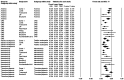
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin's lymphoma (NHL), representing 30% of all lymphoma cases. Within the first 2-3 years following immunochemotherapy, 30-40% of patients will experience a relapse or a refractory disease, thereby exhibiting a poor prognosis. High-dose immunotherapy followed by autologous stem cell transplantation is the standard care for relapsed/refractory (RR) patients with DLBCL. However, >60% of patients are ineligible for a transplant, presenting a therapeutic challenge. Chimeric antigen receptor (CAR) T-cell therapy has shown promising efficacy in patients with DLBCL, including those with R/R disease. The present study conducted a meta-analysis that showed highly favorable outcomes [objective response rate (ORR): 69%; complete remission (CR): 49%] in B-cell NHL patients (n=419) who were treated with second-generation CAR T cells. The response rate varied in different types of B-cell NHL. In 306 patients with R/R DLBCL eligible for rate evaluation, the ORR and CR rate mean estimates were 68% [95% confidence interval (CI), 55-79%] and 46% (95% CI, 38-54%), respectively. Thus, the findings indicated that immunotherapy with CAR T cells has improved outcomes for patients with R/R DLBCL and other subtypes of B-cell NHL compared with standard chemotherapy regimens. The study revealed that grade ≥3 anemia (34%) and thrombocytopenia (30%) were the most common adverse effects of CAR T-cell therapy. Incidence of grade ≥3 cytokine release syndrome and neurotoxicity associated with CAR T-cell therapy was effectively managed.
Keywords: B-cell non-Hodgkin's lymphoma; chimeric antigen receptor T cells; chimeric antigen receptor T-cell efficacy; chimeric antigen receptor T-cell safety; chimeric antigen receptor T-cell therapy; diffuse large B-cell lymphoma.
Copyright: © Al-Mansour et al.
Figures




References
-
- Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, MacPherson N, O'Reilly S, Spinelli JJ, Sutherland J, et al. Introduction of combined CHOP plus rituximab therapy dramatically improved outcome of diffuse large B-cell lymphoma in British Columbia. J Clin Oncol. 2005;223:5027–5033. doi: 10.1200/JCO.2005.09.137. - DOI - PubMed
-
- Coiffier B. Rituximab in the treatment of diffuse large B-cell lymphomas. Semin Oncol. 2002;29:30–35. - PubMed
-
- Habermann TM, Weller EA, Morrison VA, Gascoyne RD, Cassileth PA, Cohn JB, Dakhil SR, Woda B, Fisher RI, Peterson BA, Horning SJ. Rituximab-CHOP versus CHOP alone or with maintenance rituximab in older patients with diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:3121–3127. doi: 10.1200/JCO.2005.05.1003. - DOI - PubMed
LinkOut - more resources
Full Text Sources
