A COVID-19 human viral challenge model. Learning from experience
- PMID: 32790065
- PMCID: PMC7578316
- DOI: 10.1111/irv.12797
A COVID-19 human viral challenge model. Learning from experience
Abstract
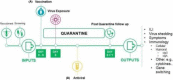
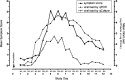
The controlled human infection model and specifically the human viral challenge model are not dissimilar to standard clinical trials while adding another layer of complexity and safety considerations. The models deliberately infect volunteers, with an infectious challenge agent to determine the effect of the infection and the potential benefits of the experimental interventions. The human viral challenge model studies can shorten the time to assess the efficacy of a new vaccine or treatment by combining this with the assessment of safety. The newly emerging SARS-CoV-2 virus is highly contagious, and an urgent race is on to develop a new vaccine against this virus in a timeframe never attempted before. The use of the human viral challenge model has been proposed to accelerate the development of the vaccine. In the early 2000s, the authors successfully developed a pathogenic human viral challenge model for another virus for which there was no effective treatment and established it to evaluate potential therapies and vaccines against respiratory syncytial virus. Experience gained in the development of that model can help with the development of a COVID-19 HVCM and the authors describe it here.
Keywords: COVID-19; RSV; SARS-CoV-19; controlled human infection model; human viral challenge model.
© 2020 The Authors. Influenza and Other Respiratory Viruses Published by John Wiley & Sons Ltd.
Conflict of interest statement
This is a review article, and all various declarations have been made on the relevant papers by the authors and cited amongst the references. However, the authors have conducted CHIM/HVCM studies and therefore declare the following for clarity. Robert Lambkin‐Williams is an Independent Consultant Virologist with the consulting company VirologyConsult Ltd. UK. He previously worked at Retroscreen Virology Ltd, which was renamed hVIVO Services Limited. This company was a Contract Research Organisation specialised in respiratory viruses and in particular the human viral challenge model. He still owns some shares in this company and does provide some consulting services to them. He provides consulting services in the area virology to other companies as an independent advisor. John DeVincenzo received RSV‐related research contracts (through the University of Tennessee) and consultancy fees from Janssen, Reviral, Pulmocide, ADMA Biologics, Ark, Pfizer and MedImmune/AstraZeneca, and has received consultancy fees from VirBio and Enanta.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

