Late onset infectious complications and safety of tocilizumab in the management of COVID-19
- PMID: 32790075
- PMCID: PMC7436682
- DOI: 10.1002/jmv.26429
Late onset infectious complications and safety of tocilizumab in the management of COVID-19
Abstract
Background: Tocilizumab (TCZ) has been used in the management of COVID-19-related cytokine release syndrome (CRS). Concerns exist regarding the risk of infections and drug-related toxicities. We sought to evaluate the incidence of these TCZ complications among COVID-19 patients.
Methods: All adult inpatients with COVID-19 between 1 March and 25 April 2020 that received TCZ were included. We compared the rate of late-onset infections (>48 hours following admission) to a control group matched according to intensive care unit admission and mechanical ventilation requirement. Post-TCZ toxicities evaluated included: elevated liver function tests (LFTs), GI perforation, diverticulitis, neutropenia, hypertension, allergic reactions, and infusion-related reactions.
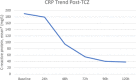
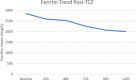
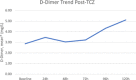
Results: Seventy-four patients were included in each group. Seventeen infections in the TCZ group (23%) and 6 (8%) infections in the control group occurred >48 hours after admission (P = .013). Most infections were bacterial with pneumonia being the most common manifestation. Among patients receiving TCZ, LFT elevations were observed in 51%, neutropenia in 1.4%, and hypertension in 8%. The mortality rate among those that received TCZ was greater than the control (39% versus 23%, P = .03).
Conclusion: Late onset infections were significantly more common among those receiving TCZ. Combining infections and TCZ-related toxicities, 61% of patients had a possible post-TCZ complication. While awaiting clinical trial results to establish the efficacy of TCZ for COVID-19 related CRS, the potential for infections and TCZ related toxicities should be carefully weighed when considering use.
Keywords: Il-6 inhibition; SARS coronavirus; coronavirus; cytokine/chemokine; immune responses; tocilizumab.
© 2020 Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures
References
-
- Chen X, Zhao B, Qu Y, et al. Detectable serum SARS‐CoV‐2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL‐6) level in critically ill COVID‐19 patients [published online ahead of print April 17, 2020]. Clin Infect Dis. 2020:ciaa449. https://pubmed.ncbi.nlm.nih.gov/32301997/ - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

