Transcriptional regulators of the Golli/myelin basic protein locus integrate additive and stealth activities
- PMID: 32790717
- PMCID: PMC7446974
- DOI: 10.1371/journal.pgen.1008752
Transcriptional regulators of the Golli/myelin basic protein locus integrate additive and stealth activities
Abstract
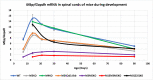
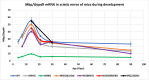
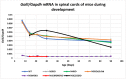
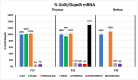
Myelin is composed of plasma membrane spirally wrapped around axons and compacted into dense sheaths by myelin-associated proteins. Myelin is elaborated by neuroepithelial derived oligodendrocytes in the central nervous system (CNS) and by neural crest derived Schwann cells in the peripheral nervous system (PNS). While some myelin proteins accumulate in only one lineage, myelin basic protein (Mbp) is expressed in both. Overlapping the Mbp gene is Golli, a transcriptional unit that is expressed widely both within and beyond the nervous system. A super-enhancer domain within the Golli/Mbp locus contains multiple enhancers shown previously to drive reporter construct expression specifically in oligodendrocytes or Schwann cells. In order to determine the contribution of each enhancer to the Golli/Mbp expression program, and to reveal if functional interactions occur among them, we derived mouse lines in which they were deleted, either singly or in different combinations, and relative mRNA accumulation was measured at key stages of early development and at maturity. Although super-enhancers have been shown previously to facilitate interaction among their component enhancers, the enhancers investigated here demonstrated largely additive relationships. However, enhancers demonstrating autonomous activity strictly in one lineage, when missing, were found to significantly reduce output in the other, thus revealing cryptic "stealth" activity. Further, in the absence of a key oligodendrocyte enhancer, Golli accumulation was markedly and uniformly attenuated in all cell types investigated. Our observations suggest a model in which enhancer-mediated DNA-looping and potential super-enhancer properties underlie Golli/Mbp regulatory organization.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

