Hydroxychloroquine and tocilizumab therapy in COVID-19 patients-An observational study
- PMID: 32790733
- PMCID: PMC7425928
- DOI: 10.1371/journal.pone.0237693
Hydroxychloroquine and tocilizumab therapy in COVID-19 patients-An observational study
Abstract
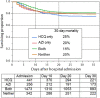
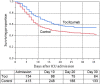
Hydroxychloroquine has been touted as a potential COVID-19 treatment. Tocilizumab, an inhibitor of IL-6, has also been proposed as a treatment of critically ill patients. In this retrospective observational cohort study drawn from electronic health records we sought to describe the association between mortality and hydroxychloroquine or tocilizumab therapy among hospitalized COVID-19 patients. Patients were hospitalized at a 13-hospital network spanning New Jersey USA between March 1, 2020 and April 22, 2020 with positive polymerase chain reaction results for SARS-CoV-2. Follow up was through May 5, 2020. Among 2512 hospitalized patients with COVID-19 there have been 547 deaths (22%), 1539 (61%) discharges and 426 (17%) remain hospitalized. 1914 (76%) received at least one dose of hydroxychloroquine and 1473 (59%) received hydroxychloroquine with azithromycin. After adjusting for imbalances via propensity modeling, compared to receiving neither drug, there were no significant differences in associated mortality for patients receiving any hydroxychloroquine during the hospitalization (HR, 0.99 [95% CI, 0.80-1.22]), hydroxychloroquine alone (HR, 1.02 [95% CI, 0.83-1.27]), or hydroxychloroquine with azithromycin (HR, 0.98 [95% CI, 0.75-1.28]). The 30-day unadjusted mortality for patients receiving hydroxychloroquine alone, azithromycin alone, the combination or neither drug was 25%, 20%, 18%, and 20%, respectively. Among 547 evaluable ICU patients, including 134 receiving tocilizumab in the ICU, an exploratory analysis found a trend towards an improved survival association with tocilizumab treatment (adjusted HR, 0.76 [95% CI, 0.57-1.00]), with 30 day unadjusted mortality with and without tocilizumab of 46% versus 56%. This observational cohort study suggests hydroxychloroquine, either alone or in combination with azithromycin, was not associated with a survival benefit among hospitalized COVID-19 patients. Tocilizumab demonstrated a trend association towards reduced mortality among ICU patients. Our findings are limited to hospitalized patients and must be interpreted with caution while awaiting results of randomized trials. Trial Registration: Clinicaltrials.gov Identifier: NCT04347993.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: DAB, SMB, and NSB are employed by Berry Consultants LLC which provided statistical support for the study. EH, AHG, ALP, SM, and SLG are employed or have ownership interest in COTA Inc which provided statistical support for the study. AHG discloses consulting fees from Physicians’ Education Resource, LLC for his contributions to COVID-19 and Cancer Care. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. https://www.covid19treatmentguidelines.nih.gov/. Accessed [May 12, 2020]. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

