Portable and accurate diagnostics for COVID-19: Combined use of the miniPCR thermocycler and a well-plate reader for SARS-CoV-2 virus detection
- PMID: 32790779
- PMCID: PMC7425953
- DOI: 10.1371/journal.pone.0237418
Portable and accurate diagnostics for COVID-19: Combined use of the miniPCR thermocycler and a well-plate reader for SARS-CoV-2 virus detection
Abstract
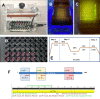
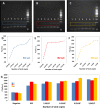
The coronavirus disease 2019 (COVID-19) pandemic has crudely demonstrated the need for massive and rapid diagnostics. By the first week of July, more than 10,000,000 positive cases of COVID-19 have been reported worldwide, although this number could be greatly underestimated. In the case of an epidemic emergency, the first line of response should be based on commercially available and validated resources. Here, we demonstrate the use of the miniPCR, a commercial compact and portable PCR device recently available on the market, in combination with a commercial well-plate reader as a diagnostic system for detecting genetic material of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causal agent of COVID-19. We used the miniPCR to detect and amplify SARS-CoV-2 DNA sequences using the sets of initiators recommended by the World Health Organization (WHO) for targeting three different regions that encode for the N protein. Prior to amplification, samples were combined with a DNA intercalating reagent (i.e., EvaGreen Dye). Sample fluorescence after amplification was then read using a commercial 96-well plate reader. This straightforward method allows the detection and amplification of SARS-CoV-2 nucleic acids in the range of ~625 to 2×105 DNA copies. The accuracy and simplicity of this diagnostics strategy may provide a cost-efficient and reliable alternative for COVID-19 pandemic testing, particularly in underdeveloped regions where RT-QPCR instrument availability may be limited. The portability, ease of use, and reproducibility of the miniPCR makes it a reliable alternative for deployment in point-of-care SARS-CoV-2 detection efforts during pandemics.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

