Delivering HDAC over 3 or 5 days as consolidation in AML impacts health care resource consumption but not outcome
- PMID: 32790847
- PMCID: PMC7448603
- DOI: 10.1182/bloodadvances.2020002511
Delivering HDAC over 3 or 5 days as consolidation in AML impacts health care resource consumption but not outcome
Abstract
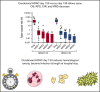
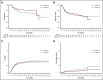
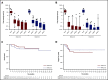
Postremission treatment is crucial to prevent relapse in acute myeloid leukemia (AML). High-dose cytarabine delivered every 12 hours on days 1, 3, and 5 (HDAC-135) is the standard of care for younger adult patients with AML. Although this standard has been unsuccessfully challenged by other treatment regimens, including multiagent chemotherapy, the timing of HDAC administration has attracted little attention. Here, we retrospectively compared the safety, efficacy, and health care resource consumption associated with HDAC-135 and another standard, condensed HDAC-123 regimen, as consolidation treatment in younger AML patients in first complete response. This study included 221 patients (median age, 46.6 years; range, 18-60 years). HDAC-123 and HDAC-135 were used in 92 and 129 patients, respectively. Both regimens were associated with similar rates of relapse-free survival, cumulative incidence of relapse, nonrelapse mortality, and overall survival, including in core binding factor AML subgroup in which levels of minimal residual disease reduction were similar in both schedules. Hematological recovery times regarding neutrophils and platelets were significantly shorter in patients receiving HDAC-123, with an average difference of 3 to 4 days for each consolidation cycle. The total duration of hospitalization for the whole postremission program was shorter with HDAC-123 (32 days; interquartile ratio [IQR], 22.0,36.5) compared with HDAC-135 (41 days; IQR, 30.5, 50.0) (P < .0001). In conclusion, the condensed HDAC-123 regimen induced faster hematological recovery and therefore significantly reduced the length of hospital stay without affecting treatment response or outcome in younger AML patients.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: P.-Y.D. has served on advisory boards for Daiichi-Sankyo and Astellas. S.B. has served on advisory boards for Daiichi-Sankyo, Astellas, Sanofi, and Jazz Pharmaceuticals. A.B. has served on advisory boards for Daiichi-Sankyo and Novartis. A.P. has received research grants to his institution from Incyte, Janssen, Gilead, Sanofi, Amgen, Novartis, Celgene, Jazz Pharma, Daiichi-Sankyo, Astellas, Roche; and served on advisory boards for Sanofi, Takeda, AbbVie, Janssen, Jazz Pharma, Daiichi-Sankyo, Astellas, Novartis, Celgene, Otsuka, Sunesis, Roche, and Pfizer. C.R. has received research grants to his institution from AbbVie, Amgen, Novartis, Celgene, Jazz Pharma, Agios, Daiichi-Sankyo, Astellas, Sunesis, Roche, and MaatPharma; and served on advisory boards for AbbVie, Janssen, Jazz Pharma, Daiichi-Sankyo, Astellas, Novartis, Celgene, Otsuka, Sunesis, Roche, Otsuka, Macrogenics, and Pfizer. The remaining authors declare no competing financial interests.
Figures
References
-
- Mayer RJ, Davis RB, Schiffer CA, et al. ; Cancer and Leukemia Group B . Intensive postremission chemotherapy in adults with acute myeloid leukemia. N Engl J Med. 1994;331(14):896-903. - PubMed
-
- Skipper HE, Schabel FM Jr., Wilcox WS. Experimental evaluation of potential anticancer agents. XIII. On the criteria and kinetics associated with “curability” of experimental leukemia. Cancer Chemother Rep. 1964;35:1-111. - PubMed
-
- Schaich M, Röllig C, Soucek S, et al. . Cytarabine dose of 36 g/m2 compared with 12 g/m2 within first consolidation in acute myeloid leukemia: results of patients enrolled onto the prospective randomized AML96 study. J Clin Oncol. 2011;29(19):2696-2702. - PubMed
-
- Burnett AK, Russell NH, Hills RK, et al. . Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol. 2013;31(27):3360-3368. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

