Epithelial Cell Migration and Proliferation Patterns During Initial Wound Closure in Normal Mice and an Experimental Model of Limbal Stem Cell Deficiency
- PMID: 32790859
- PMCID: PMC7441334
- DOI: 10.1167/iovs.61.10.27
Epithelial Cell Migration and Proliferation Patterns During Initial Wound Closure in Normal Mice and an Experimental Model of Limbal Stem Cell Deficiency
Abstract
Purpose: Establishing the dynamics of corneal wound healing is of vital importance to better understand corneal inflammation, pathology, and corneal regeneration. Numerous studies have made great strides in investigating multiple aspects of corneal wound healing; however, some aspects remain to be elucidated. This study worked toward establishing (1) if epithelial limbal stem cells (LSCs) are necessary for healing all corneal wounds, (2) the mechanism by which epithelial cells migrate toward the wound, and (3) if centrifugal epithelial cell movement exists.
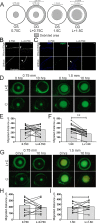
Methods: To establish different aspects of corneal epithelial wound healing we subjected mice lacking hyaluronan synthase 2 (previously shown to lack LSCs) and wild-type mice to different corneal debridement injury models.
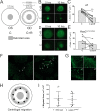
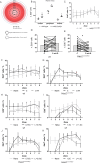
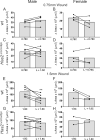
Results: Our data show that both LSCs and corneal epithelial cells contribute toward closure of corneal wounds. In wild-type mice, removal of the limbal rim delayed closure of 1.5-mm wounds, and not of 0.75-mm wounds, indicating that smaller wounds do not rely on LSCs as do larger wounds. In mice shown to lack LSCs, removal of the limbal rim did not affect wound healing, irrespective of the wound size. Finally, transient amplifying cells and central epithelial cells move toward a central corneal wound in a centripetal manner, whereas central epithelial cells may move in a centrifugal manner to resurface peripheral corneal wounds.
Conclusions: Our findings show the dimensions of the corneal wound dictate involvement of LSCs. Our data suggest that divergent findings by different groups on the dynamics of wound healing can be in part owing to differences in the wounding models used.
Conflict of interest statement
Disclosure:
Figures

References
-
- Thoft RA, Friend J. The X, Y, Z hypothesis of corneal epithelial maintenance. Invest Ophthalmol Vis Sci. 1983; 24: 1442–1443. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

